Pharmacology & Toxicology Poster Session
| INABIS '98 Home Page | Your Session | Symposia & Poster Sessions | Plenary Sessions | Exhibitors' Foyer | Personal Itinerary | New Search |
Introduction
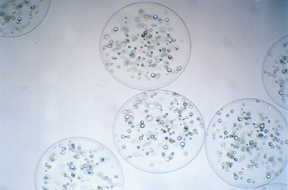 Figure 1. Alginate-polylysine-alginate(APA) Microcapsules containing cells. The outer circle is the capsular membrane which surrounds the cells on the inside.
Figure 1. Alginate-polylysine-alginate(APA) Microcapsules containing cells. The outer circle is the capsular membrane which surrounds the cells on the inside.
Gene therapy may be defined as a treatment for genetic or non-genetic diseases involving the delivery of a therapeutic gene. Gene therapy can be classified as either ex vivo gene therapy, where a patient's cells are modified outside of their body, or in vivo gene therapy where the patient's cells are modified in the body using a variety of viral vectors, liposomal vectors or naked DNA. Our lab is developing an alternative form of gene therapy called somatic gene therapeutics. Somatic gene therapeutics involves encapsulating a universal cell line that has been engineered to secrete a therapeutic protein, and implanting the resulting microcapsules into a patient who is deficient in that particular protein. In the patient, the encapsulated cells will continue to secrete the therapeutic protein while protected from the immune system by the microcapsule.
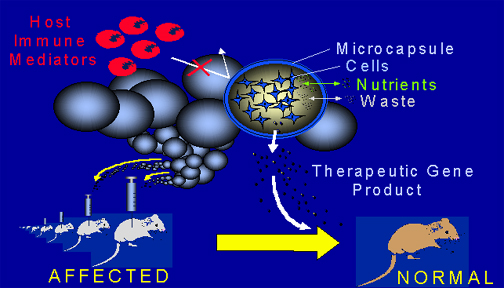 Figure 2. Diagrammatic Overview of Somatic Gene Therapeutics. The same cell line can be encapsulated and implanted into all of the patients with the same disease. The capsules protect the encapsulated cells from the immune system.
Figure 2. Diagrammatic Overview of Somatic Gene Therapeutics. The same cell line can be encapsulated and implanted into all of the patients with the same disease. The capsules protect the encapsulated cells from the immune system.
There are several advantages to our form of gene therapy. First, it is relatively easy to perform. While ex vivo gene therapy requires the manipulation of every patients cells individually, somatic gene therapeutics makes use of the same universal cell line for implantation into all of the patients with the same disease. Treatment involves a simple intra-peritoneal injection of microcapsules. Second, somatic gene therapeutics is safe. Since their creation, people have been concerned about the possible adverse effects of using viral vectors for gene therapy especially those that are randomly inserted into the genome. On the other hand, somatic gene therapeutics does no modify the patients cells or genome and if cells somehow escape the microcapsule they will be immediately destroyed by the body's immune system. Third, somatic gene therapeutics has the advantage of being reversible. Other approaches to gene therapy modify the patient's cells or DNA and thus are essentially irreversible if a problem arises. However, somatic gene therapeutics does not alter the patient's cells in any way and if a problem should arise the capsules can be immediately retrieved. Fourth, repeated treatments are possible with somatic gene therapeutics. Conversely, many viral gene therapy protocols are only effective for the first administration after which the body's immune system is activated and can clear any new virus particles. Last, viral gene therapy strategies are limited by the size of DNA that the vector can hold while somatic gene therapeutics is not restricted by the size of gene involved.
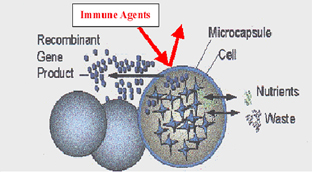 Figure 3. The importance of pore size for somatic gene therapeutics. The pore size must be large enough to allow for the diffusion of nutrients, wastes and therapeutic proteins but small enough to exclude agents of the immune system.
Figure 3. The importance of pore size for somatic gene therapeutics. The pore size must be large enough to allow for the diffusion of nutrients, wastes and therapeutic proteins but small enough to exclude agents of the immune system.
The success of somatic gene therapeutics is dependent on four critical factors:
Biocompatability: the capsules must be biocompatable to prevent their rejection by the immune system when they are implanted. The outer layer of alginate is responsible for the biocompatability of the microcapsules.
Secretion in vivo: it is necessary to have a cell line which is secreting a therapeutic protein and these cells must be able to survive inside of the implanted microcapsule and continue to secrete therapeutic protein. This is dependent of the pore size of the capsules which is primarily determined by the polylysine coating. The pores must be large enough to allow for diffusion of nutrients, waste and therapeutic protein.
Immunoisolation: the capsules must be able to prevent agents of the immune system from entering the microcapsule and attacking the non-autologous encapsulated cells. Again, the pore size is primarily determined by the polylysine coating and in this case it must be smaller than the immune agents to exclude them from the interior of the capsule.
Structural Stability: the capsules must remain intact during the implantation procedure and while they are present in the patient. If the capsule is broken, the encapsulated cells will be exposed to the immune system and and this will lead the death of those cells as well as the sensitization of the immune system.
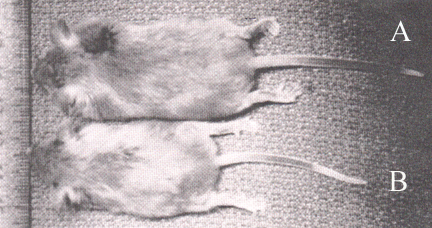 Figure 4. Representative Snell Dwarf Mice treated with microcapsules containing either mouse growth hormone secreting cells(A) or non-secreting cells(B). Clearly, mouse A shows an increase in size due to the delivery of mouse growth hormone from the encapsulated cells.
Figure 4. Representative Snell Dwarf Mice treated with microcapsules containing either mouse growth hormone secreting cells(A) or non-secreting cells(B). Clearly, mouse A shows an increase in size due to the delivery of mouse growth hormone from the encapsulated cells.
| <= Abstract | INTRODUCTION | Materials & Methods => |
| Discussion Board | Next Page | Your Poster Session |