Poster Contents
| INABIS '98 Home Page | Your Session | Symposia & Poster Sessions | Plenary Sessions | Exhibitors' Foyer | Personal Itinerary | New Search |
Introduction
In both human hypertension and animal models of hypertension, receptor-mediated vasodilation is impaired for systems linked to activation of adenylyl cyclase. This parallels a defect in receptor-stimulated adenylyl cyclase activity.
Human and animal studies have linked this defect in receptor-stimulated adenylyl cyclase to impaired receptor/G-protein coupling.
Using human lymphocytes as a model we have recently demonstrated that G-protein-coupled receptor kinase (GRK) expression was increased in lymphocytes from hypertensive subjects paralleling the decrease in beta-adrenergic-stimulated adenylyl cyclase activity.
The present studies were performed to determine:
1)Whether the increase in GRK expression in lymphocytes fromhypertensive subjects is due to increased GRK mRNA levels.
2)Whether the increase in lymphocyte GRK expression is paralleled by an increase in vascular smooth muscle GRK.
Materials and Methods
Subject protocol
We recruited 14 normotensive and 7 borderline/mildly hypertensive subjects. All subjects were male Caucasian and drug free and instructed to maintain a high sodium diet for three days before study. On the morning of the study, subjects were admitted to the Clinical Investigation Unit at LHSC-UC. An intravenous catheter was inserted into an antecubital vein and 20 minutes later a 120 ml blood sample was obtained.
Animal protocol
Male spontaneously hypertensive (SHR) and normotensive Wistar rats (220-350 g, Charles River) were used. Indirect tail-cuff measurements of systolic blood pressure were obtained in lightly anesthetized rats using a Harvard rat tail monitor. In all SHRs studied systolic blood pressure was > 120 mmHg (mean SBP 134+/-1 mmHg) and in all Wistar rats studied, systolic blood pressure was <110 (mean SBP 94+/-2 mmHg).
Lymphocytes
Lymphocytes from human and rats were separated from EDTA anticoagulated whole blood by the method of Boyum. Cytosolic fractions for assessment of G-protein-coupled receptor kinase activity were obtained by nitrogen cavitation (Parr bomb, 600 psi, 15 minutes 4°C). For assessment of adenylyl cyclase activity, lymphocytes were permeabilized with digitonin in Hanks' balanced salt solution.
Isolated vascular smooth muscle cells
Freshly isolated thoracic aortae were extensively cleaned and dissociated into isolated vascular smooth muscle cells with collagenase and papain.
Assessment of GRK activity
GRK enzymatic activity was assessed in lymphocyte cytosolic fraction via the extent of light-dependent phosphorylation on rhodopsin.
Assessment of GRK protein expression
GRK protein expression was determined by immunoblotting with a specific mouse monoclonal antibody 3A10, raised against purified recombinant bovine GRK-2.
Assay of adenylyl cyclase activity
Assays of adenylyl cyclase activity were performed in permeabilized lymphocytes. Adenylyl cyclase activity was assessed by the conversion of [32P]ATP to [32P]cAMP. [32P] cAMP was separated from [32P]ATP by sequential dowex and alumina chromatography.
Northern blot analysis
Total RNA was isolated from human lymphocytes using the TRIzol method (GibcoBRL). GRK-2 mRNA expression was determined on the membrane using a 718-bp Sac I open-reading frame of bovine GRK-2. GAPDH mRNA expression was determined on the same membrane after being stripped with boiling 0.1% SDS.
Assessment of tension in aortic ring segments
Aortic ring segments (2-3 mm wide) were suspended in Krebs'physiological salt solution under optimal tension in individual double-walled organ baths maintained at 37°C and gassed with 5% CO2/ 95% O2. The rings were equilibrated for 60 minutes. Contractile responses were measured using isometric force-displacement transducers. Following equilibration, rings were maximally pre-constricted with phenylephrine (5 uM) and allowed to plateau. Relaxation was assessed in response to single addition of isoproterenol (10 uM) or sodium nitroprusside (10 nM). Relaxation response was quantitated by the area above the curve.
Results
Blood pressures were significantly higher in the borderline/mildly hypertensive subjects as compared to the normotensive controls (systolic blood pressure: hypertensive 135+/-3 mmHg; normotensive 123+/-1 mmHg; diastolic blood pressure: hypertensive 89+/-2 mmHg; normotensive 74+/-2 mmHg).
Adenylyl cyclase actviity in human lymphocytes
Isoproterenol-stimulated adenylyl cyclase activity was significantly reduced in lymphocytes from hypertensive subjects (Figure 1A). In contrast forskolin-stimulated adenylyl cyclase activity was not significantly altered (Figure 1B).
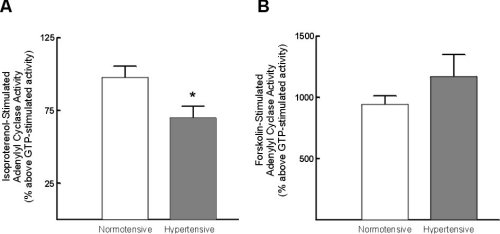
Figure 1:Alterations in lymphocyte adenylyl cyclase activity with
hypertension. A) Alterations in isoproterenol-stimulated adenylyl cyclase
activity; B) alterations in forskolin-stimulated adenylyl cyclase activity.
Data represent mean +/- standard error of mean. * = p < 0.05 vs normotensive.
GRK activity and protein expression in human lymphocytes
Light-dependent phosphorylation of rhodopsin was significantly increased (33+/-9%) in cytosolic fractions from hypertensive subjects as compared to normotensive controls (Figure 2A). Immunodetectable GRK-2 was also significantly increased (55+/-7%)in hypertensive subjects (Figure 2B).
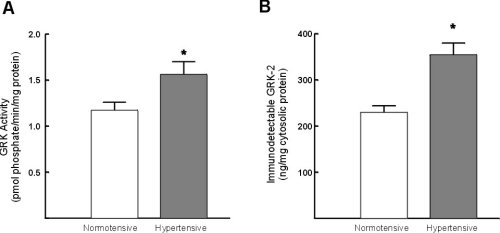
Figure 2:Assessment of GRK activity and GRK-2 protein expression in lymphocytes from normotensive and hypertensive subjects. A) Assessment of GRK activity in lymphocyte cytosolic fractions. The extent of GRK-mediated phosphorylation was determined in triplicate. B) Assessment of GRK-2 protein expression in whole cell pellets from hypertensive and normotensive subjects. The data represent the mean +/- standard error of the mean. * = p < 0.05 vs normotensive control subjects.
GRK-2 protein expression was significantly inversly correlated with isoproterenol-stimulated adenylyl cyclase activity (i.e. those subjects with the highest GRK-2 expression had lowest beta-adrnergic stimulated response, Figure 3A). Additionally, GRK-2 protein expression was significantly correlated with systolic blood pressure (Figure 3B).
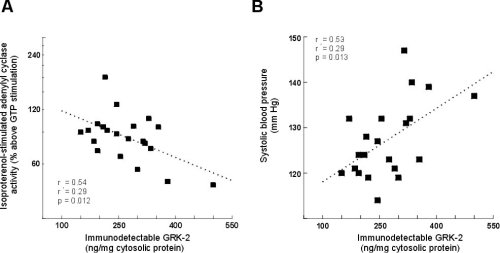
Figure 3:The correlation between GRK-2 expression and isoproterenol-stimulated
adenylyl cyclase activity (A) and systolic blood pressure (B) in younger
normotensive and hypertensive subjects.
Assessment of GRK mRNA expression
The GRK-2 probe identified a 3.8 Kb fragment consistent with labelling of GRK-2 mRNA. Steady state GRK-2 mRNA levels were not significantly increased in lymphocytes from hypertensive subjects (Figure 4).
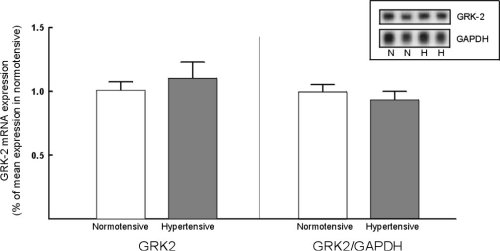
Figure 4:GRK-2 mRNA expression in normotensive and hypertensive subjects.
Left panel: GRK-2 mRNA expression normalized for the average expression
in lymphocytes from normotensive subjects. Right panel: GRK-2 mRNA expression
normalized for GAPDH mRNA expression. Inset: representative northern blot
of GRK-2 and GAPDH expression in normotensive (N) and hypertensive (H)
subjects.
Adenylyl cyclase activity in rat lymphocytes
Isopoterenol-stimulated adenylyl cyclase activity was significantly reduced in lymphocytes from SHR as compared to Wistar rats, to an extent comparavle to that seen in borderline/hypertensive human subjects (Figure 5A). In contrast, forskolin-stimulated adenylyl cyclase activity was not significantly altered (Figure 5B).
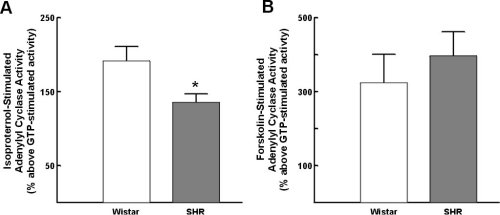
Figure 5:Alterations in lymphocyte adenylyl cyclase in Wistar (normotensive)
rats and spontaneously hypertensive rats (SHR). A) alterations in isoproterenol-stimulated
adenylyl cyclase activity; B) alterations in forskolin-stimulated adenylyl
cyclase activity. Data represent the mean +/- standard of the mean. * =
p<0.05.
Alterations in GRK-2 protein expression in lymphocytes and vascular smooth muscle cells
Immunodetectable GRK-2 protein expression in lymphocytes from SHR was increased by 43+/-10% as comapred to Wistar rats (Figure 6A). Further, a similar increase in GRK-2 protein expression was found comparing lymphocytes from SHR vs WKY strain (a 31+/-11% increase in SHR lymphocytes). In vascular smooth muscle cells GRK-2 protein expression was increased 69+/-14% comparing SHR versus Wistar rats (Figure 6B). A comarable increase in GRK-2 protein expression was found comparing SHR to WKY strain (an 83+/-8% increase).
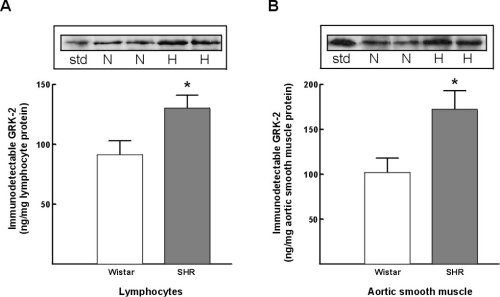
Figure 6:Alterations in GRK-2 protein expression in lymphocytes and vascular smooth muscle cells from Wistar (normotensive) and SHR (hypertensive) rats. A) lymphocyte GRK-2 expression. B) Vascular smooth muscle GRK-2 expression. Insets representative autoradiographs of Western blots from lymphocytes (left) and vascular smooth muscle cells (right) in normotensive Wistar rats (N) and in spontaneously hypertensive rats (H). The extent of expression was determined utilizing concomitant GRK-2 standards (std).
Discussion and Conclusion
1)GRK-activity and GRK-2 protein content are increased in lymphocytes from hypertensive subjects. This increase parallels a reduction in lymphocyte beta-adrenergic-stimulated adenylyl cyclase activity.
2)Increased GRK-2 protein content is not due to an increase in GRK-2 messenger RNA.
3)In SHR lymphocyte beta-adrenergic-stimulated adenylyl cyclase activity was reduced and paralleled an increase in lymphocyte GRK-2 protein content.
4) In SHR the impairment in isoproterenol-mediated vasorelaxation parallels an increase in vascular smooth muscle GRK-2 protein content.
Increased GRK-2 protein content is generalized in both lymphocytes and vascular smooth muscle cells in hypertension.
The increase in GRK-2 protein content is not due to a transcriptional mechanism.
These studies further support the hypothesis that the alterations in GRK-2 expression could underlie the defect in G-protein-coupled receptor systems, leading to the impairment in adenylyl cyclase-mediated vasodilation, characteristic of the hypertensive state.
| Discussion Board | Previous Page | Your Poster Session |