Ophthalmology Poster Session
| INABIS '98 Home Page | Your Session | Symposia & Poster Sessions | Plenary Sessions | Exhibitors' Foyer | Personal Itinerary | New Search |
Introduction
The extracellular concentration of glutamate, an excitatory neurotransmitter throughout the CNS, including the retina, must be kept low to ensure proper glutamate-mediated neurotransmission during synaptic activation and to prevent neuronal excitotoxic damage from prolonged exposure to glutamate and subsequent activation of glutamate receptors. Maintenance of extracellular glutamate is under the control of high-affinity, Na+-dependent glutamate transporters in the plasma membranes of neurons and glial cells, including glial cells in the retina. Reversed transport of glutamate, from inside the cell to outside the cell may be an important mechanism contributing to neuronal damage in a variety diseases, including stroke and glaucoma, where ischemic conditions are a factor. Release of glutamate from glial cells has been demonstrated to occur through the reversal of a glutamate transporter, but recent studies of neuronal glutamate transporters cloned from neurons and expressed in oocytes suggest that reversed transport of glutamate does not occur in the neuronal glutamate transporter.
A further example of the functional differences between neuronal and glial cell glutamate transporters is that dysfunctional glial cell transporters, rendered dysfunctional by transgenic methods, mediate glutamate neurotoxcity in the brain, while dysfunctional neuronal transporters contribute little to glutamate neurotoxicity. In this study we have shown that a retinal glial cell exhibits reversed transport of glutamate, releasing a large, dense cloud of extracellular glutamate surrounding retinal ganglion cells that is sufficient to mediate long-term glutamate neurotoxicity. Calcium-dependent release and reversed transport of glutamate from retinal neurons plays a relatively minor role in this release pattern.
Materials and Methods
Slices of mouse retina about 125 uM in thickness were prepared similar to that described by Werblin. The slices were maintained in oxygenated NeuralBasal Medium (Gibco) and visualized under deep red light (668 nm) using Normarski interference optics. Visible and fluorescent images of retinal slices were captured using a Santa Barbara Instrument Group (Santa Barbara, CA) model 6I cooled CCD camera. Purified rat retinal ganglion cells were prepared. Bipolar and Muller cells were isolated by papain digestion. The assay solution consisted of 10 uM glutamate dehydrogenase (GDH, Boehringer Mannheim, USA) and 10 uM glutamate pyruvate transaminase (GPT, Sigma, USA) that was added to the recording chamber in the NeuralBasal Medium (Gibco). In the presence of glutamate, NAD in the solution was converted by GDH to NADH, and glutamate to alpha-ketoglutarate. Radiation (lamda= 360 nm) from a Hg arc lamp was used to excite NADH, and the resulting fluorescence was localized with the CCD camera.
Results
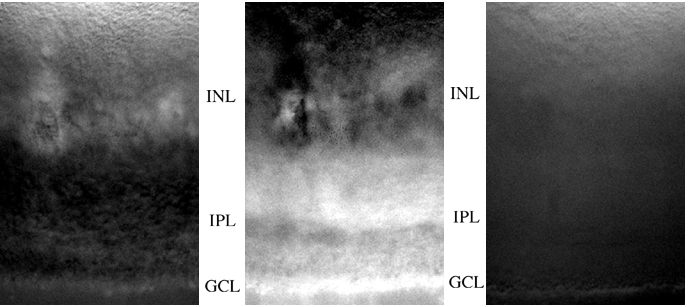 Figure 1
Figure 1
Using this flurometric assay for glutamate, coupled with the CCD camera, the release of glutamate from mouse retinal slices was observed. Figure 1 contains images from a representative retinal slice. The left panel reveals the glutamate release under basal (no stimulation) conditions. The center panel is when the retinal slice was depolarized with 40 mM external potassium in the bathing medium. Under this condition, a large amount of glutamate can be seen coming from the inner plexiform layer (IPL; presumably from bipolar cells) and over the ganglion cell layer (GCL). The right panel depicts glutamate release when the glutamate transport blocker THA is added to the elevated potassium medium. In this case, most of the IPL release and all of the GCL release is curtailed.
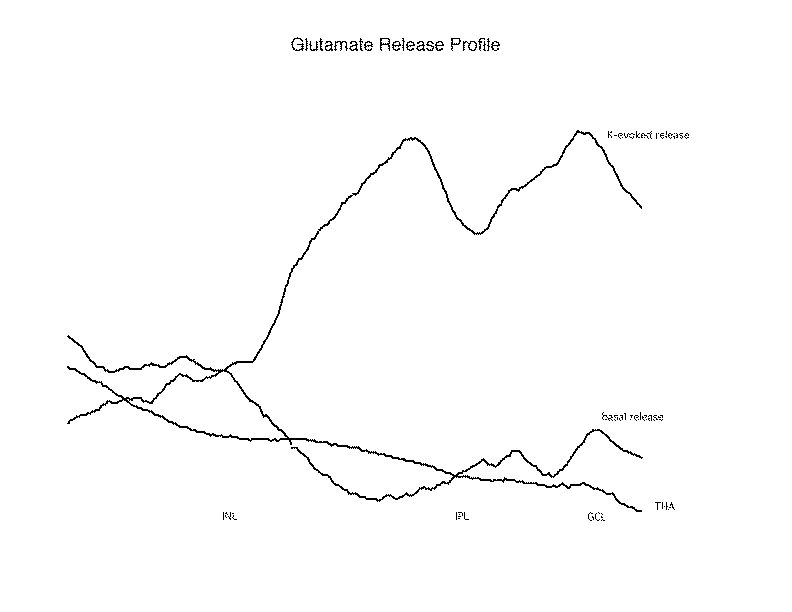 Figure 2
Figure 2
A profile of the glutamate release is prepared to condense the date from each collection of fluorescence images. This is accomplished by collapsing the image to a line of single pixels, each containing the average intensity of the horizontal line of pixels from the image. When this is done, one can observe the amounts of glutamate release from the successive layers of the retina under different conditions. As seen in this Release Profile, potassium evoked a large release in the IPL and GCL, and this was blocked by THA. Of interest, the THA blocked not only the K-evoked release from the GCL, but also the basal release observed in that area, indicating that this basal release may also have a transporter dependent origin.
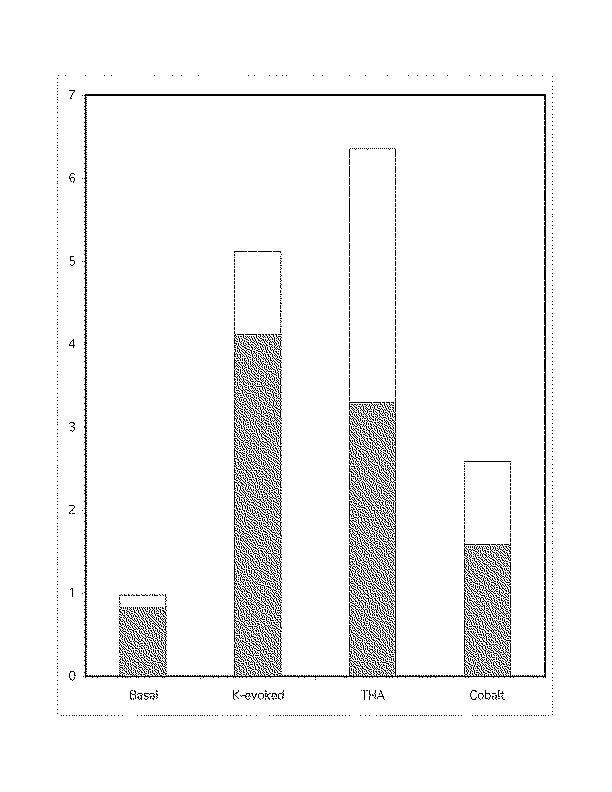 Figure 3
Figure 3
Using isolated retinal cells, we examined glutamate release from individual cells under similar conditions as above. Again, the release was observed under basal conditions, K-evoked situations, THA addition, and finally 2 mM cobalt was added to block calcium-dependent release. The cells examined all showed some glutamate release, with the largest amount under K-evoked conditions. While THA had little effect on bipolar (Fig 3a) and ganglion cells (Fig 3b), it suppressed half of the K-evoked release from Müller cells (Fig 3c). Cobalt, which diminished the bipolar and ganglion cell release, had no effect on the Müller cells. The values on the ordinate are millimolar glutamate concentrations.
Discussion and Conclusion
1. Endogenous glutamate is released from mouse retina via calcium-dependent and calcium-independent mechanisms.
2. There is a significant K-evoked glutamate release into the ganglion cell layer. This release is carried via a glutamate transporter and is principally not calcium-dependent.
3. Isolated retinal bipolar cells, ganglion cells and Müller cells release endogenous glutamate upon depolarization.
4. The glutamate released from bipolar cells and ganglion cells is primarily calcium-dependent, while the release from the Müller cells is via the glutamate transporter.
5. The transporter mediated glutamate release from Müller cells appears to be the source of glutamate released into the ganglion cell layer.
References
1. Faden, A.I., Demediuk, P., Painter, S.S., Vink, R. Science 244, 798 (1989).
2. D. W. Choi, Neuron 1, 623 (1988)
3. B.K. Siesjo, News Physiol Sci. 5, 120 (1990).
4. M. Szatkowski and D. Attwell, Trends. Neurosci. 17, 359 (1994).
5.Szatkowski, M., Barbour, B., Attwell, D. Nature 348:443-446 (1990).
6. Zerangue, N. Kavanaugh, MP, Nature 383:634-637 (1996).
7. Maguire, G., Simko, H., Weinreb, R.N. and Ayoub, G.S. J. Neurosci. (Abstr) (1997).
8. Vorwerck, C.K., Lipton, S.A., Zurakowski, D., Hyman, B.T., Sabel, B.A. and Dryer, E.B. Invest. Ophthalmol. Vis. Sci. 37:1618-1624 (1996)
9. Massey, S.C. and Maguire, G. In: Excitatory Amino Acids and Synaptic Transmission. London, Academic Press (1997), pp201-221.
10. Attwell, D. and Mobbs, P. Curr. Opinion Neurobiol. 4:353-359 (1994).
11. Brew, H. and Attwell, D. Nature 327: 707-709 (1987
12. Rothstein, J.D., Dykes-Hoberg, M., Pardo, C.A., Bristol, L.A., Kuncle, R., Kanai, Y., Hediger, M.A., Wang, Y., Schielke, J.P. and Welty, D.F. Neuron 16: 675-686 (1996).
13. Tanaka, T. et al. Science 276: 1699-1702 (1997).
14. Kaneko, A., Pinto, L.H. and Tachibana, M. J. Physiol. 410: 613-629 (1989).
15. Lagnado, L., Gomis, A. and Job, C. Neuron 17: 957-967 (1996)
16. Marc, R.E., Liu, S., Kallonitis, M., Raiguel, S.F., Van Haesendonck, E. J. Neurosci. 10: 4005-4034 (1990).
17. Newman, E. and Reichenbach, A. Trends Neurosci. 19: 307-312 (1996).
18. Dryer, E.B., Zurakowski, D., Schumer, R.A., Podos, S.M. and Lipton, S.A. Arch. Ophthalmol. 114: 299-305 (1996)
19. Ambati, J. et al. Arch Ophthalmol. 115:1161-1166.
20. Tanihara, H., Hangai, M., Sawaguchi, S., Abe, H., Kageyama, M., Nakazawa, F., Shirasawa, E. and Honda, Y. Arch. Ophthalmol. 115: 752-756.
21. Ekstrom, P., Sanyal, S., Narfstrom, K., Chader, G.J., van Veen, T. Invest. Ophthalmol. Vis. Sci. 29: 1363-1371 (1988).
22. Erickson, P.A., Fisher, S.K., Guerin, C.J., Anderson, D.H.and Kaska, D.D. Exp. Eye Res. 44: 37-48 (1987).
23. Linser, P.J., Sorrentino, M. and Moscona, A.A. Dev. Brain Res. 13: 65-71 1984).
24. Werblin, F.S. J. Physiology 294:613-626 (1978).
25. Lindsey, J.D. and Weinreb, R.N. Invest. Ophthalmol. Vis. Sci. 35: 3640-3648 (1994).
26. Maguire, G. and Werblin, F. J. Neurosci. 14: 6094-6101 (1994).
| Discussion Board | Previous Page | Your Poster Session |