Occupational Health - Public Health Poster Session
| INABIS '98 Home Page | Your Session | Symposia & Poster Sessions | Plenary Sessions | Exhibitors' Foyer | Personal Itinerary | New Search |
Introduction
A large number of epidemiological and experimental studies were focused in the last 10 years on the implications of lead exposure on several essential elements homeostasis: calcium, magnesium, copper, zinc, iron and their quantitative relation in different organs /3, 4, 5, 6, 10, 12, 13/.
The relationship between lead on one side and copper and zinc on other side was demonstrated at the level of many body compartments. This paper aims to establish the way in which, in experimental exposure to lead, its action can modify the levels of copper and zinc in the body of tested animals. It is followed as well the emphasize of some relationship between the levels of copper and zinc, lead dose and exposure time.
Materials and Methods
White Wistar male rats, aged 45 days were chosen for this experiment. Their initial body weight was 120 ± 10 g. The animals were kept in laboratory conditions and drinking water was deionizated and offered ad libidum.
Animals were exposed to lead via ingestion: aqueous solution of lead acetate 2% and respectively 20%, daily, for 15 and 30 days in quantity of 0.1 ml per 100 g. body weight. The solution was prepared from distillated water. To avoid the circadian variations of studied parameters, the administration of toxic and sacrifices were done at the same hour. Animals were sacrificed by decapitation after ether anesthesia. Serum copper and zinc were made by spectrophotometry in flame atomization with a Perkin Elmer 300 device.
Animals were separated in 5 groups: group 1 (10 control animals with distillated water); group 2 (8 animals intoxicated with lead acetate 2% during 15 days); group 3 (8 animals intoxicated with lead acetate 2% during 30 days); group 4 (8 animals intoxicated with lead acetate 20% during 15 days); group 5 (8 animals intoxicated with lead acetate 20% during 30 days).
Results
The animals belonging to the 2, 3, 4 and 5 groups showed a smaller weight increase than those from the control group. The medium weight from group 2 was 116.4 g., smaller than that of the control group 147.1 g. at the end of the experiment (p<0.001). At groups 3 and 5 medium weight at sacrifice was 141 respectively 145 g. and at control group was 161 g. (p<0.01).
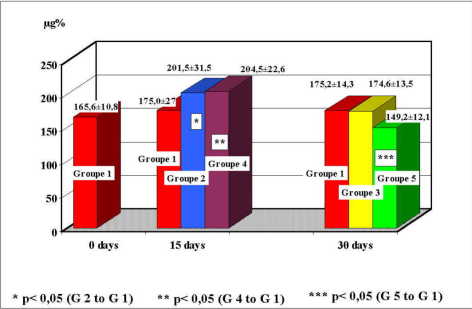
At group 2, medium values of blood copper were 201.5 ± 31.5 µg%, values significantly increased comparatively to the control group which were 175 ± 27.2 µg% (p<0.05). The group 3 had medium values of blood copper of 174.6 ± 13.5 µg%, close to those of control group 175.2 ± 14.3 µg%. At group 4, medium values of blood copper were 204.5 ± 22.6 µg%, values significantly increased comparatively to the control group which were 175 ± 27.2 µg% (p<0.05). The group 5 had values of 149.2 ± 12.1 µg%, significantly decreased comparatively to the control group which were 175.2 ± 14.3 µg% (p<0.05)(figure 1).
 Click to enlarge
Fig. 1: Distribution of blood copper values at study groups.
Click to enlarge
Fig. 1: Distribution of blood copper values at study groups.
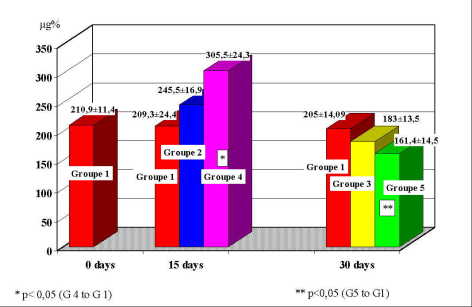
At group 2, medium values of blood zinc were 245.5 ± 16.9 µg%, values increased comparatively to the control group which were 209.3 ± 24.41 µg%. The group 3, sacrificed at 30 days, had medium values of blood zinc of 183.3 ± 13.5 µg%, significantly decreased comparatively to those of group 2 (sacrificed at 15 days). At group 4, medium values of blood zinc were 305.5 ± 24.5 µg%, values significantly increased comparatively to the control group which were 209.3 ± 24.4 µg% (p<0.005). The group 5 had values of 161.4 ± 14.5 µg%, significantly decreased comparatively to the group 4 (sacrificed at 15 days) and to the control group which were 205 ± 14.9 µg% (p<0.05)(figure 2).
 Click to enlarge
Fig. 2: Distribution of blood zinc values at study groups.
Click to enlarge
Fig. 2: Distribution of blood zinc values at study groups.
Discussion and Conclusion
The evolution of the animals' weight during the 30 days of the experiment showed a decrease in weight gain at animals intoxicated with lead. This finding appeared in other studies too and is due, at least partially, to the nutritional deficiencies induced by the administration of the toxic via the digestive tract. This explanation is also supported by the evolution of total serum proteins, which was descending too.
During the experiment, the concentration of zinc evolved in two different phases. After 15 days, we had seen a dose dependent increase of blood level higher for the group with greater dose of lead. After 30 days it has been ascertained a decrease with high statistically significance for the group with higher dose of lead, both with control at 15 and 30 days.
Making an attempt to discuss this phenomenon, we must refer to the experimental studies of Miller /7, 8/, who concludes that lead makes an alteration of zinc distribution between different tissues and organs with a significant decrease of levels in brain, kidneys and bones, and an increase in liver and pancreas. As far as the zinc elimination through urine is concerned this process is different depending on the lead dose: at small doses the urinary zinc rate elimination is proportional to that of lead, while at large doses it becomes constant plate /14/. It is known that dietary zinc deficiency cause a lead redistribution with accumulation in blood, bones and cerebellum /2/. The opposite phenomenon was also noted: the increase of the dietary zinc causes the decrease of lead concentration in blood, liver and kidney and at the same time the decrease of urinary elimination of deltaaminolevulinic acid, decrease of eritrocitar protoporphirine, recovery of eritrocitar dehidrase of deltaaminolevulinic acid and inhibition of lead intestinal absorption /1/. On the other hand, it is known that zinc is a constitutive part of deltaaminolevulinic acid dehidrase, enzyme which is the zone of lead major attack /11/. The basic mechanism of this enzyme inhibition is blocking by competition of zinc by toxic /11/.
In the light of these data, our findings lead to the presumption that in the first phase there is an increase of blood zinc as an effect of biological active zinc "internal depletia", and then there is a massive increase of zinc elimination with consecutive decrease of blood zinc.
Blood copper had a similar evolution with zinc, with increases at 15 days and important decreases at 30 days, highly significant compared to previous results and stage controls. Decrease of blood copper was noticed 20 years ago by Sporn/12/, but the author did not mention the two stage evolution of blood copper because the sacrification had only one stage. The correlation between lead and copper can also be deducted from the finding that copper dietary deficiency favours the retention of lead, and supplementation of copper ratio protects against lead toxicity. The two phase evolution of blood copper could be due to the alteration of physiological distribution time of copper in tissues/7/.
CONCLUSIONS
1. An alteration of copper and zinc homeostasis is done by lead poisoning.
2. The behavior in time of blood copper and zinc levels has two phases, early increase of the two elements levels being followed by a subsequent significant decrease. The phenomenon can be explained by a possible redistribution in successive phases of these elements between different target organs under the action of lead.
References
1. ANCA ZOE, OSSIAN A., KOVACS A., IVANESCU G. Concentratia Pb, Zn si Cu din sangele si creierul de sobolan tratat cu Pb si Pb + Se.
Rev. Igiena, vol. XXXVII, nr. 5, p. 439-444, 1988.
2. ASHRAF M. H., FOSMIRE G. T. Effects of marginal zinc deficiency on subclinical lead toxicity in the rat neonate.
Rev. Am. Inst. Of Nutrition, 1985.
3. BOSCOLO P., CARNIGIANI M., CARELLI G., FINELLI V. N., GIULIANO G. Zinc and copper in tissues of rats with blood hypertension induced by long term lead exposure.
Arch. of Env. Contamination and Toxicol., 21(1), 72-77, Iul. 1991.
4. FINELLI V. H., EL GAZZAR R. M. Interaction of lead and zinc on the prothrombin activity in rats.
Toxicol. Letters, 1, 33-39, 1977.
5. FLORA S. I., KUMAR D., DASGUPTA S. Interaction of zinc, methionine or their combination with leab at gastrointestinal or post-absorbtive level in rats.
Pharmacol. and Toxicol., 68(1), 3-7, Ian. 1991.
6. GOYER R. A. Lead toxicity: current concerns (Review).
Env. Health Perspectives, 100: 177-187, Apr. 1993.
7. MILLER G. D., MASSARO T. F., KOPEREK E. Low level lead exposure and the zinc-dependent-organ tissue. Distribution of essential elements in the neonatal rats.
Biol. Trace Elem. Research, 6, 35-38, 1984.
8. MILLER G. D., MASSARO T. F., MASSARO E. J. Interactions between lead and essential elements. A review,
Neurotoxicology, 11(1), 99-119 Spring,1990.
9. OSSIAN A. Cercetari cu privire la valoarea testelor biochimice în aprecierea gradului intoxicatiei profesionale cu plumb.
Teza de doctorat, Fac. de Medicina Cluj-Napoca, 1982.
10. PANEMANGALORE M., BEBE F. N. Effects of low oral lead and cadmium exposure and zinc status of heme metabolites in weanlingvats.
Int. I. of Occup. Med. and Environ. Health, 9(2), 141-151, 1996.
11. SCHLIPKOTTER H. W., GELERTER L., OST B. Investigations on the combined effects of zinc and lead.
Zbl. Nakt. Hugg. I. Abt. Orig. B, 60, 130-138, 1975.
12. SPORN A., CARSTEA A. Contributie la stabilirea actiunii concomitente a arsenului, cuprului, zincului, plumbului si staniului. Toxicitatea acuta.
Rev. Igiena, vol. XVIII, nr. 2, 1969.
13. TRUCKENBRODT R., WINTER L., SCHALER K. H. Effect of occupational lead exposure on various elements in the human blood. Effects of calcium, cadmium, iron, copper, magnesium, manganese and zinc levels in the human blood erythrocytes and plasma in vivo.
Zentralblatt Fur Bakteriologie, mikrobiologie und Hygiene -1- abt - Originale B, Hygiene 179(3), 187-197, Ian. 1984.
14. VICTERY V., MILLER C. R., SHI-YA ZHU, GOYER R. A. Effect of different levels and periods of lead exposure on tissue levels and excretion of lead, zinc and calcium in the rat.
Fundam. and Appl. Toxicol, 8, 506-516, 1987.
| Discussion Board | Previous Page | Your Poster Session |