Invited Symposium: Signal Transduction in Endothelium: Mechano-Sensing, Ion Channels and Intracellular Calcium
| INABIS '98 Home Page | Your Session | Symposia & Poster Sessions | Plenary Sessions | Exhibitors' Foyer | Personal Itinerary | New Search |
Introduction
In endothelial cells an increase in intracellular free Ca2+ concentration is the main mediator for autcoid-induced nitric oxide (NO) formation. Evidence was provided that the agonist-stimulated biosynthesis of NO by the membrane bound/associated NO synthase (eNOS) stricly depends on extracellular Ca2+ (Lückhoff et al., 1988), indicating that an influx of Ca2+ via so called capacitative Ca2+ entry is essential for NO production. In contrast, intracellular Ca2+ release via either inositol 1,4,5-trisphosphate (IP3) or the SERCA inhibitor, thapsigargin, are much less active to induce NO formation, although (in the case of thapsigargin) an prolonged elevation of the bulk Ca2+ concentration ([Ca2+]b) is observed even in the absence of extracellular Ca2+.
Recently we could demonstrate that in endothelial cells a subplasmalemmal Ca2+ control unit (SCCU) exists, which provides a localized Ca2+ signaling in the subplasmalemmal area insulated from the perinuclear cytosol (Graier et al., 1998). Evidence was provided that endoplasmic reticulum ryanodine-sensitive Ca2+-induced Ca2+ release (RSCR) mechanism in association with cell membrane Na+/Ca2+ exchange proteins (Na/CaX) contribute to the function of the SCCU. In the present study we used endothelial cells freshly isolated from bovine left circumflex coronary arteries to investigate whether RsCR indeed occurs in the subplasmalemmal area in response to autacoid stimulation. Hence the role of RsCR for in agonist-induced intracellular Ca2+ release, CCE and activation of eNOS was studied (this work of our group has been published recently: Paltauf-Doburzynska et al., 1998).
Materials and Methods
Cell isolation and culture: Endothelial cells were isolated from bovine left circumflex coronary arteries as described previously (Graier et al., 1992; Graier et al., 1995).
Ca2+ measurement: Intracellular free Ca2+ concentration was determined in single endothelial cells using microfluorometry (Graier et al., 1995). As described previously (Graier et al., 1998), different Ca2+ sensitive dyes were used to monitor perinuclear free Ca2+ concentration ([Ca2+]peri ; fura-2) and the subplasmalemmal Ca2+ concentration ([Ca2+]sub ; FFP-18; Davies & Hallett, 1996; Etter et al., 1996). For fura-2 loading, the cells were incubated in the dark with 2 µmol l-1 fura-2/am in DMEM for 45 min. The cell membrane impermeable dye FFP-18 was loaded using the laser stress wave loading technique (Graier et al., 1998). For measurement of Ca2+ extrusion, cells were resuspended in nominal Ca2+-free Hepes-buffered solution containing 1 µmol l-1 fura dextran potassium salt (MW 70,000).
Data acquisition: Single cell [Ca2+]peri was recorded in a microfluorometer, which excited alternatively with 360 and 380 nm, while emission was detected at 510 nm using photon counting technique (Graier et al., 1995; Sturek et al., 1991). For measurements of [Ca2+]sub, fluorescence of FFP-18 at 335/365 nm excitation and 500 nm emission was monitored in suspended cells every 0.2 s in a spectrofluorometer (Hitachi F-4500). Extrusion of Ca2+ was measured with fura dextran by monitoring extracellular Ca2+ concentration at 340 and 364 nm excitation and 500 nm emission.
Digital confocal microscopy: Images were obtained with a scientific-grade CCD camera using conventional lamp illumination (Graier et al., 1998; Paltauf-Doburzynska et al., 1998). Out-of-focus fluorescence was calculated with a computer algorithm using the three-dimensional point spread function for each microscope objective (Waters & Brown, 1996; Carrington et al., 1995). The instrumental setup includes a liquid-cooled CCD camera (Quantix, Photometrics, Munich, Germany) on an inverted microscope (Nikon Eclipse TE300, Nikon, Vienna, Austria) with z-stage motor controlled by the Ludl-z-stage control box (Ludl, Inc., Fairfield Imaging, Turnbridge Wells, UK). Images were collected with a CFI Plan Fluor 100x oil immersion objective (N.A.=1.3; 0.068 µm/pixel, Nikon, Vienna, Austria). Out-of-focus fluorescence was calculated with Micro Tome® deconvolution software (VayTek, Inc., Fairfield Imaging, Turnbridge Wells, UK) using the advanced constrained iterative deconvolution algorithm (5 iterations). 3-dimensional reconstruction was performed with VoxBlast® (VayTek, Inc., Fairfield Imaging, Turnbridge Wells, UK).
Measurements of eNOS activity: Activity of eNOS was determined by measuring the conversion of [3H]-L-arginine to [3H]-L-citrulline (Mayer et al., 1991) as described previously (Graier et al., 1996).
Results
1. Using digital confocal microscopy fura-2 was found throughout the whole cytosol, while FFP-18 was exclusively in the cell membrane (Fig.1). Thus, bulk ([Ca2+]b) and subplasmalemmal ([Ca2+]sub) Ca2+ concentration were monitored using fura-2 and FFP-18.
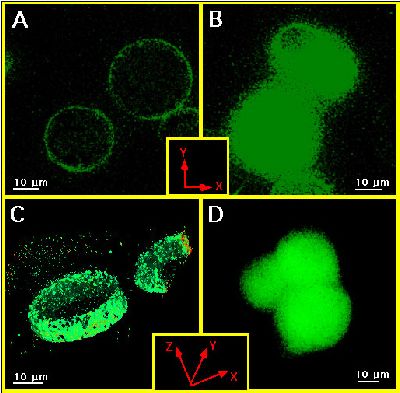 Figure 1
Figure 1
2. Initiating RsCR by 200 nmol l-1 ryanodine increased [Ca2+]sub, while [Ca2+]b remained constant. However, when Na/CaX was prevented, ryanodine was able to elevate [Ca2+]peri.
3. Blockage of RsCR by 25 ymol l-1 ryanodine diminished Ca2+ extrusion to stimulation with Bk in normal Na+ containing solution (Fig.2). These data indicate that under control conditions RsCR occurs in response to autacoid stimulation in the subplasmalemmal area and that the Ca2+ released by RsCR is pumped out via Na/CaX.
4. Inhibition of RsCR diminished capacitative Ca2+ entry in response to bradykinin (Fig.3).
5. Direct activation of RsCR failed to activate eNOS. However, an inhibition of RsCR diminished the effect of ATP and Bk on eNOS, while the effect of thapsigargin remained unchanged (Fig.4).
Discussion and Conclusion
Our data provide evidence that during autacoid stimulation RsCR occurs in the subplasmalemmal area. This release is insulated from deeper cytosolic compartments. The Ca2+ released by the RsCR in response to a stimulus is pumped out of the cell via Na/CaX. Although RsCR, under control conditions, does not contribute to elevation of [Ca2+]b, it is involved in the activity (but not activation) of the capacitative Ca2+ entry. These findings support our previous observations that in endothelial cells a subplasmalemmal Ca2+ signaling occurs controlled by an subplasmalemmal Ca2+ control unit (SCCU; Graier et al., 1998). Here we have characterized RsCR and Na/CaX as the main proteins involved in this spatial and insulated Ca2+ signaling. Moreover, we have extended the function of the SCCU not only to control [Ca2+]sub but to the regulation of eNOS activity.
NOTE: An extensive discussion is available at: 1. Graier et al., 1998 2. Paltauf-Doburzynska et al., 1998
References
- Carrington, W.A., Lynch, R.M., Moore, E.D.W., Isenberg, G., Fogarty, K.E. & Fay, F.S. (1995). Superresolution three-dimensional images of fluorescence in cells with minimal light exposure. Science 268: 1483-1487.
- Davies, E.V. & Hallett, M.B. (1996). Near membrane Ca2+ changes resulting from store release in neutrophils: detection by FFP-18. Cell Calcium 19: 355-362.
- Etter, E.F., Minta, A., Poenie, M. & Fay, F.S. (1996). Near-membrane [Ca2+] transients resolved using the Ca2+ indicator FFP18. Proc. Natl. Acd. Sci. USA 93: 5368-5373.
- Graier, W.F., Paltauf-Doburzynska, J., Hill, B., Fleischhacker, E., Hoebel, B.G., Kostner, G.M. & Sturek, M. (1998). Submaximal stimulation of porcine endothelial cells causes focal Ca2+ elevation beneath the cell membrane. J. Physiol. Lond. 506: 109-125.
- Lückhoff, A., Pohl, U., Mülsch, A. & Busse, R. (1988). Differential role of extra- and intracellular calcium in the release of EDRF and prostacyclin from cultured endothelial cells. Br J Pharmacol 95: 189-186.
- Mayer, B., John, M. & Böhme, E. (1991). Partial purification and characterization of a Ca2+/calmodulin-dependent endothelium-derived relaxing factor-forming enzyme from porcine cerebellum. J. Cardiovasc. Pharmacol. 17(Suppl.3): S46-S51.
- Paltauf-Doburzynska, J., Posch, K., Paltauf, G. & Graier, W.F. (1998). Stealth ryanodine-sensitive Ca2+ release contributes to activity of capacitative Ca2+ entry and nitric oxide synthase in bovine endothelial cells. J. Physiol. Lond. 513: 369-379.
- Waters, D. & Brown, C. (1996). New high-resolution 3-D microscope - Avoids damage to live samples. Biophotonics International 9/10: 40-44.
| Discussion Board | Previous Page | Your Symposium |