Neuropharmacology Poster Session
| INABIS '98 Home Page | Your Session | Symposia & Poster Sessions | Plenary Sessions | Exhibitors' Foyer | Personal Itinerary | New Search |
Introduction
The anxiolytic, anticonvulsant and other therapeutic actions of BZs are thought to be mediated by central-type benzodiazepine (BZ) receptors ( CBRs), which are coupled to type A gamma-aminobutyric acid (GABA A) receptors in the CNS. Several variants of the separate subunits of the GABA A -CBR complex have been cloned, indicating the complex heterogeneity exhibited by this GABA-gated chloride ionophore. Although the subunit and ligand-binding stoichiometries await clarification, expression studies indicate that a fully functional GABA A -CBR receptor requires a combination of alpha, beta and gamma subunits. There is also evidence that the type of subunit present in the receptor complex determines the pharmacological characteristics of the CBR (Sieghart, 1994).
BZs also interact with peripheral-type BZ receptors ( PBRs) which are found in diverse peripheral tissues and also in the CNS. Subcellular localization studies have shown that PBR sites are primarily present on the outer mitochondrial membrane. These sites are also referred to as mitochondrial BZ receptors (MBR), and are involved in the regulation of cellular metabolism and steroidogenesis.Using the isoquinoline carboxamide, PK14105, for photoaffinity labelling, an 18 kD isoquinoline binding protein (IBP) has been localized in diverse tissues that express MBRs . Purification studies have revealed that, in addition to the IBP, the MBR also consists of a 30 kDa adenine nucleotide carrier (ADC) and a 32 kDa.voltage-dependent anion channel (VDAC). There is no appreciable homology between the cloned IBPs and the cloned GABA A receptor subunits, indicating that the PBR and the CBR are structurally distinct and unrelated receptors (Parola et al., 1993).
In addition to their interaction with the above classical CBRs and PBRs, there is increasing evidence that BZs act on G protein- coupled receptors to alter adenylyl cyclase activity in the CNS, as described below .
Materials and Methods
3H- Diazepam(83 Ci/mmol) and 32 P- GTP (3,000 Ci/mmol) were purchased from Mandel. 32 P- ATP (3,000 Ci/mmol) and 3H- cAMP were from Amersham and ICN, respectively. All other drugs and reagents were obtained from Sigma.
Animals: Male Sprague-Dawley rats weighing about 300g were maintained at a room temperature of 22o C, with food and water freely available. Animals were decapitated and striata dissected on ice for binding and functional assays.
Receptor Binding:Fresh striatal tissue was homogenized by polytron for 5s in 50 vol of 50mM Tris-HCL buffer ( pH 7,4 at 4o C ). After centifugation at 39,000g for 10 min and two washes in the same buffer, homogenates were resuspended in 50 mM Tris-HCL ( pH 7.4 at 30o C ) at a concentration of about 5mg/ml ( wet weight/ vol ). Binding assays were carried out at 30o C for 60 min with 3 H- Diazepam ( 0.25- 83 nM ), with or without 1mM GTP. Non-specific binding was determined in the presence of 10uM diazepam.
Adenlyl Cyclase Assay: Fresh striatal tissue was hand- homogenized in cold 50 mM HEPES /NaOH buffer containing 5 mM MgCl 2 1mM dithiothreitol, and 0.2 mM EGTA (pH 7.4 at 30o C). The homogenate was centrifuged at 39,000g for 10 min, and membranes were washed twice in the same buffer. AC activity was measured as the conversion of 32 P- ATP to 32 P- cAMP , in the presence or absence of forskolin and other drugs, as described previously (Tenn et al., 1996 ).
GTP Binding : Agonist- stimulated 32 P- GTP binding was measured as described by Friedman et al. (1993 ). Membranes ( ~200ug protein per assay tube) were incubated for 2 min at 30o C in assay buffer. After addition of 50nM 32 P- GTP with or without diazepam, the incubation continued for 5 min. Incubates were centrifuged at 10,000g for 15 min at 4o C, pellets were resuspended in sample buffer and incubated at room temperature for 1 h with gentle shaking. Aliquots containing 20ug protein were analyzed by SDS- PAGE and 32 P- GTP- labeled proteins were quantified by densitometry ( Tenn and Niles, 1997 ).
Results
Effect of GTP on Diazepam Binding: Control binding parameters were: KD = 19.3 + 0.8 nM ; BMAX = 632 + 21 fmol/mg protein, n = 3. In the presence of GTP , there was a modest but significant ( p <.05 ) decrease in binding affinity, but no change in receptor density: KD = 30.5 + 2.6 nM ; BMAX = 688 + 77 fmol/mg protein, n = 3.
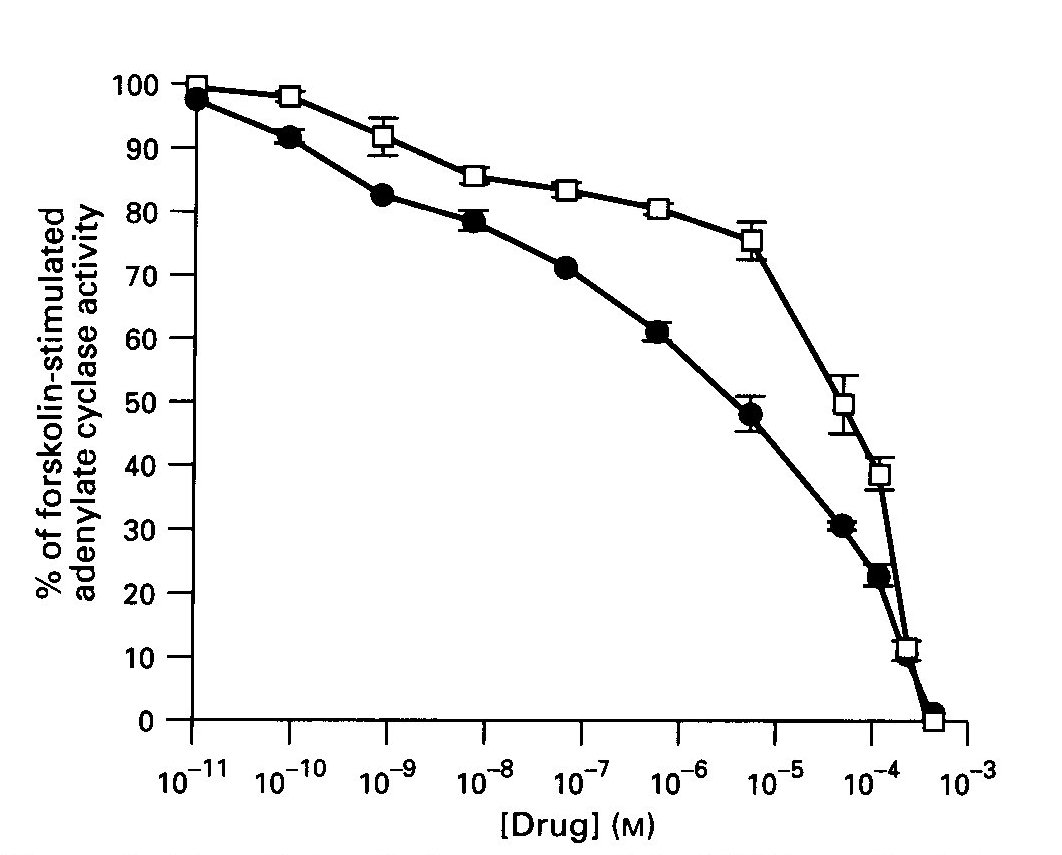
Effect of Benzodiazepines on AC Activity: The BZ agonists, diazepam and Ro5-4864, caused a concentration- dependent inhibition of forskolin-stimulated AC activity in the striatum, as shown in Fig. 1. An F- test indicated a significantly better fit to two-site and three- site models for Ro5-4864 and diazepam, respectively. The first receptor-mediated phase of inhibition by Ro5-4864 occurred with an EC 50 = 1.36nM (0.64-2.08 ), whereas the second phase had an EC 50 = 131uM (87.9-174 ). Diazepam was more potent, and inhibited enzyme activity with an EC 50 = 0.43 nM for the first phase and 1.0 uM (0.68-1.32) and 194 uM for the other phases.
Fig.1: The effects of diazepam and Ro5-4864 on forskolin-stimulated adenylate cyclase (AC) activity in rat striatum. Diazepam ( solid circles), Ro5-4864 ( open squares). Data are the means + SE. of 3-4 experiments performed in duplicate.
Effects of BZ Antagonists on BZ Signalling: The central- type BZ antagonist, flumazenil (1uM), did not block the inhibitory effect of Ro5-4864 on forskolin-stimulated AC activity ; but, in fact, enhanced this effect at lower agonist concentrations (10-11- 10 -10 M, p< 0.001 vs Ro5-4864 without flumazenil). In contrast, the peripheral - type receptor agent, PK11195 (5 uM), blocked the first receptor- mediated phase of inhibition by Ro5-4864, but did not affect the second phase ( Fig.2).
Fig.2: The effects of benzodiazepine antagonists on Ro5-4864-induced inhibition of AC activity in striatum. Membranes were incubated with the indicated concentrations of Ro5-4864, in the absence (solid circles) or presence (open circles) of 5uM PK11195 or 1uM flumazenil ( open triangles).
Data are means + SE of 3-4 experiments carried out in duplicate. *p < .001.
Effect of Pertussis Toxin Treatment: Pretreatment of striatal tissue with pertussis toxin (8.5 ug/ml) did not affect basal or forskolin- stimulated AC activity. However, the toxin blocked the inhibitory effect of diazepam and Ro5-4864, and also that of the indoleamines, melatonin and 2-iodomelatonin, on forskolin- stimulated AC activity (Fig. 3).
Fig.3: Effect of pertussis toxin on the inhibitory action of benzodiazepines and indoleamines on stimulated AC activity. Diazepam ( 10uM), Ro5-4864 ( 50uM), Melatonin ( 500uM) and 2-iodomelatonin ( 500uM). Hatched columns, effect of drug alone; solid columns, + pertussis toxin.
Data are means + SE of 3 experiments. *p <.01, **p <.001 vs control. a, p <.005, b,p < .001 vs drug without toxin.
Effect of Diazepam on GTP Binding A 5-min incubation of striatal membranes with 10 or 50 uM diazepam induced a significant increase in 32 P GTP binding to a 40-kDa protein , as shown in Fig. 4a and b. This effect of diazepam was enhanced significantly following dopaminergic denervation of the striatum ,by 6-hydroxydopamine lesions in the substantia nigra (Fig. 4), indicating sensitization of the G-BZR-AC pathway, as reported previously (Tenn and Niles, 1997).
Fig.4: Effect of diazepam on 32 P GTP binding to a 40-kDa protein in membranes from control (C) and 6-hydroxydopamine - lesioned (L) striata. A: Representative autoradiogram of 32 P -GTP- labeled proteins separated on SDS-PAGE. B:Densitometric quantification of GTP binding to 40-kDa protein. Data are means =SD of optical density values from 2-3 experiments. Treatment x stimulation interaction, p < .001. *p < .001
vs controls ( Scheffe's test).
Discussion and Conclusion
In this study, several lines of evidence support the presence of G protein- coupled BZ receptors,which mediate the inhibitory action of BZ agonists, on the AC-cAMP pathway in the rat striatum. The multiphasic dose- response curves observed suggest that more than one mechanism is involved. The first high- affinity phase is similar to that reported for other inhibitory receptors such as the dopamine D2 receptor ( Olianas and Onali, 1987), whereas the second phase involving low (micromolar) affinity sites, is consistent with evidence that high concentrations of BZs can act directly on AC to suppress its activity ( Dan'ura et al., 1988).
The failure of flumazenil to block the inhibitory effect of Ro5-4864 on AC activity, indicates that central-type BZ receptors are not involved. In contrast, the peripheral-type receptor ligand, PK11195, blocked the first phase of inhibition by Ro5-4864, suggesting involvement of a receptor with PBR-like characteristics. Although PK 11195 has been reported to act as an agonist in some biological systems, in the absence of BZs, it did not alter basal or stimulated AC activity, which indicates that it acts as an antagonist at a G protein- coupled BZ receptor ( G-BZR).
In addition to the foregoing, the presence of a G-BZR is supported by the ability of guanine nucleotides to uncouple high- affinity diazepam binding, as reflected by a decrease in receptor affinity, in the presence of GTP. Similarly, pertussis toxin which uncouples inhibitory G protein ( G i )- associated receptors, blocked the effect of BZs and other drugs, which act on the G-BZR to inhibit AC activity. Finally, the ability of diazepam to stimulate GTP binding,to a G protein of the same size as G i , is an important functional hallmark of a G protein- coupled receptor.
In conclusion, these findings indicate that G-BZRs mediate the inhibitory effect of BZ agonists on the AC-cAMP pathway in the striatum. Given the widespread involvement of cAMP-protein kinase A ( PKA) - dependent mechanisms in the regulation of synaptic transmission in the CNS ( Colwell and Levine, 1995), it is very likely that G-BZRs are involved in mediating the neuropsychopharmacological effects of BZs and related agents.
References
- Colwell, CS, Levine, MS (1995) Excitatory synaptic transmission in neostriatal neurons: regulation of cyclic AMP- dependent mechanisms. Journal of Neuroscience, 15:1704-1713.
- Dan'ura, T, Kurokawa, T, Yamashita, Y, Yanagiuchi, H, Ishibashi, S (1988) Inhibition of rat brain adenylate cyclase activity by benzodiazepine through the effects of Gi and catalytic proteins. Life Science 42: 469-475.
- Friedman E, Butkerait, P, Wang, H.-Y. (1993) Analysis of receptor - stimulated an dbasal guanine nucleotide binding to membrane G proteins by sodium dodecyl sulfate- polyacrylamide gel electrophoresis. Analytical Biochemistry 214:171-178.
- Olianas, MC, Onali, P (1987) Pertussis toxin attenuates D2 inhibition and enhances D1 stimulation of adenylate cyclase by dopamine in rat striatum. Journal of Neurochemistry 48:1443-1447.
- Parola, AL, Yamamura, HI, Laird, HE (1993) Peripheral- type benzodiazepine receptors. Life Science 52: 1329-1342.
- Sieghart, W (1994) Pharmacology of benzodiazepine receptors: an update. Journal of Psychiatry and Neuroscience 19: 24-29.
- Tenn CC, Neu JM, Niles, LP (1996) PK11195 blockade of benzodiazepine- induced inhibition of forskolin-stimulated adeylate cyclase activity in the striatum. British Journal of Pharmacology 119:223-228.
- Tenn CC, Niles, LP (1997) Sensitization of G protein-coupled benzodiazepine receptors in the striatum of 6-hydroxydopamine-lesioned rats. Journal of Neurochemistry 69: 1920-1926.
-
| Discussion Board | Previous Page | Your Poster Session |
 click to enlarge
click to enlarge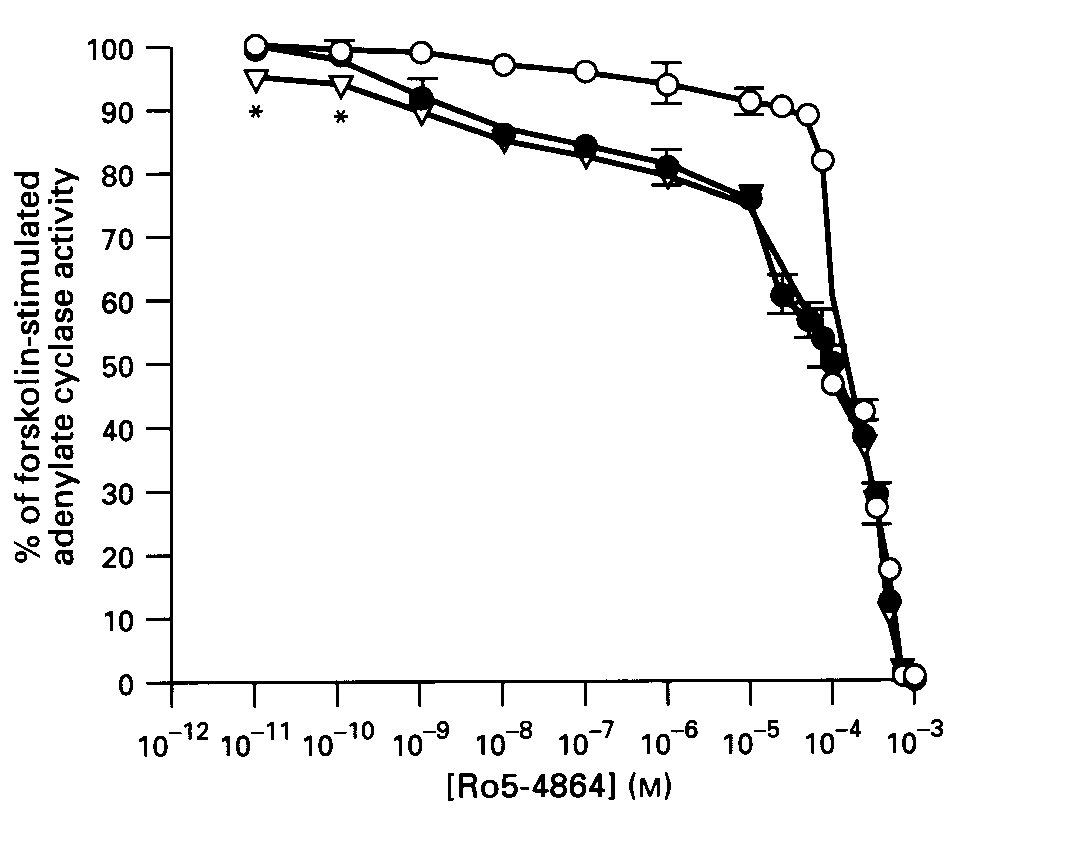 click to enlarge
click to enlarge click to enlarge
click to enlarge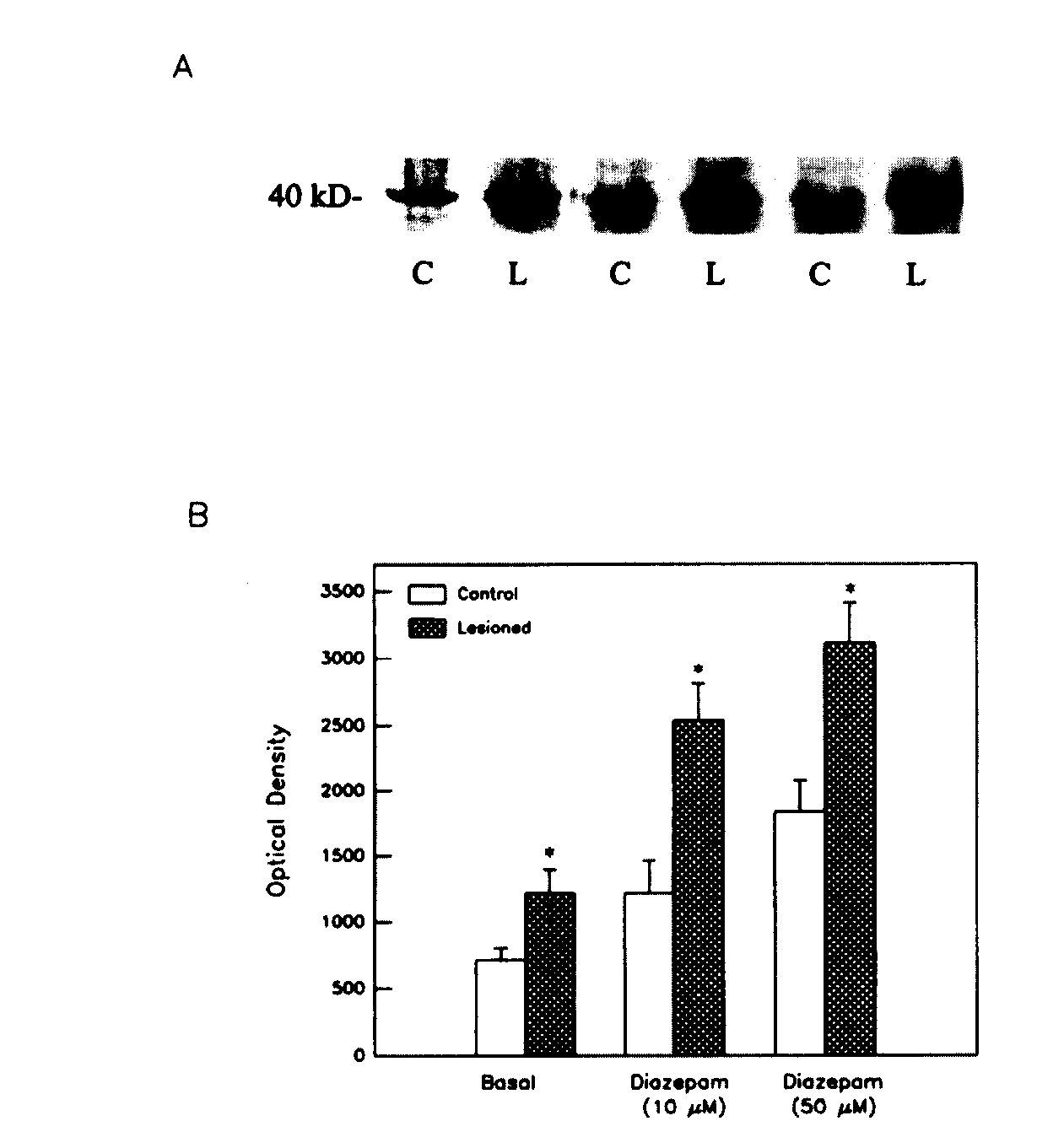 click to enlarge
click to enlarge