Invited Symposium: MAOIs: Mulptiple Effects and Sites of Action
| INABIS '98 Home Page | Your Session | Symposia & Poster Sessions | Plenary Sessions | Exhibitors' Foyer | Personal Itinerary | New Search |
Introduction
A number of biologically-active compounds that contain imidazoline or guanidinium moieties (see Fig. 1) selectively interact with a family of membrane proteins termed imidazoline binding proteins or imidazoline receptors.
The involvement of imidazoline binding proteins in the biological activities of compounds such as idazoxan, cirazoline, clonidine and amiloride remains a subject of great debate and the question is complicated by the identification of multiple potential imidazoline binding proteins. This family of membrane-associated proteins is divided into multiple types according to tissue distribution, cellular localization, apparent Mr of identified proteins and rank order of ligand binding affinity. I2 type imidazoline binding proteins are identified by binding of idazoxan, BU224 and guanabenz while I1 sites preferentially bind clonidine, have lower affinity for the guanidiniums and are present in lower abundance than I2 sites. MAO-A and -B were identified as members of the I2 family of imidazoline binding proteins by partial sequencing of imidazoline binding sites from rabbit kidney (Tesson et al., 1995) and also by labeling of the proteins in human tissues with the photoaffinity probe 2-[3-azido-4[ 125I]iodophenoxy]methyl imidazoline (AZIPI) (Lanier et al., 1993,Raddatz et al, 1995).
The imidazoline binding domain (IBD) on human MAO-B labeled by AZIPI was further localized to amino acids K149 - M222 (Raddatz et al , 1997). Although imidazoline /guanidinium compounds inhibit MAO activity, the IBD is distinct from the region of the enzyme that interacts with the MAO inhibitors such as pargyline, deprenyl and lazabemide (Cesura et al. , 1996). However, mutations within the IBD region of MAO affect enzyme activity, substrate selectivity and inhibitor selectivity suggesting that the IBD may be a novel regulatory domain on MAO.
Interestingly, the IBD is not accessible for binding of imidazoline/guanidinium compounds in all tissues that express MAO-B.
The site may be "masked" by endogenous substances (such as clonidine displacing substance (Atlas and Burstein, 1984)) known to interact with imidazoline binding proteins. Alternatively, heterogeneity within the population of MAO-B may explain the discrepancy. In the current studies, we investigated (1) the effect of imidazoline/guanidinium compounds on MAO-B activity in vitro and (2) the tissue dependent accessibility of the imidazoline binding site on MAO-B.
Materials and Methods
Membrane Preparation.
Frozen tissues were homogenized in lysis buffer [5 mM Tris HCl pH 7.5, 5 mM EDTA, 5 mM EGTA and 0.1 mM phenylmethylsulfonyl fluoride (PMSF), 4 °C] and crude membranes were prepared as previously described (Raddatz et al., 1995).
Briefly, the lysate was centrifuged at 1000 x g for 10 min at 4 °C and the resulting supernatant was centrifuged at 35,000 x g for 10 min at 4 °C .
The resulting pellet was washed and resuspended in 50 mM Tris pH 7.5 containing 0.6 mM EDTA, 5 mM MgCl2 and 0.1 mM PMSF. Membranes were rapidly frozen and stored at -70 °C.
Radioligand binding. Membranes were incubated with radioligands in the presence or absence of inhibitors for 30 - 40 min at 24 °C with shaking. Binding of [3H]-idazoxan was performed in the presence of 10 µM rauwolscine to block interaction with alpha 2-adrenergic receptors and non-specific binding was determined in the presence of 10 µM cirazoline. Binding of [3H]Ro 19-6327 was performed in the presence of 0.1 µM clorgyline to block interaction with MAO-A and non-specific binding was determined in the presence of 100 µM Ro 16-6491.
Membranes were collected by vacuum filtration on glass fiber filters (#32, Schleicher & Schuell) and washed in 4 x 3 ml of 100 mM Tris pH 7.4, 4°C. Bound radioactivity was measured with approximately 60% efficiency. Saturation and competition binding data were evaluated using the non-linear curve-fitting program LIGAND and were best fit by a one-site model.
MAO-B activity assay. Human liver membranes (30 µg protein/ tube) or platelet membranes (200 µg protein/ tube) were diluted in sodium phosphate buffer (100 mM) pH 7.4 and preincubated with inhibitors (or buffer) for 10 min at 24 °C prior to the addition of [14C]phenylethylamine (10 mM, 14 mCi/mmole). The selective MAO-B inhibitor Ro 16-6491 (100 µM) was used to define specific activity. Reactions were incubated for 20 min at 37 °C and stopped by addition of 1 ml of HCl (2N) at 4°C. The reaction products were extracted into 1 ml of ethyl acetate/toluene 1: 1 (v/v), and the radioactivity contained in an aliquot of the organic phase was measured with approximately 95% efficiency.
Immunoblots. Membrane proteins were solubilized in loading buffer (60 mM Tris-HCl, pH 6.8, containing 2 % SDS, 10 % glycerol, 1 % ß-mercaptoethanol, and 0.05 % bromophenol blue) at 100 °C for 5 min and separated on 9% SDS-polyacrylamide gels. Proteins were transferred to polyvinylidene difluoride membranes by semidry electroblotting, the blots were blocked with 5 % non fat dried milk in wash buffer (phosphate buffer saline, pH 7.5 with 0.1 % Tween 20) and probed with polyclonal antisera recognizing MAO-A and MAO-B (1:10,000). Immunoreactive proteins were visualized by chemiluminescence following incubation with peroxidase-conjugated secondary antibodies and exposure of blots to film.
Photoaffinity labeling. The cirazoline derivative, 2-(3-amino-4-iodophenoxy)methyl imidazoline was iodinated and converted to the photolabile azide ([125I]-AZIPI) for use as a photoaffinity adduct as previously described (Lanier, et al. 1993). Membranes were incubated in reduced light with 1-2 nM [125I]-AZIPI for 30 min at 24°C, chilled on ice and diluted 10-fold with 50 mM Tris buffer containing 2 mM dithiothreitol.
Samples were immediately photolyzed at 4°C for 5 min (320 nm), membranes were centrifuged, solubilized in loading buffer (see above) at 100 °C for 5 min and proteins separted by SDS-polyacrylamide gel electrophoresis.
Results
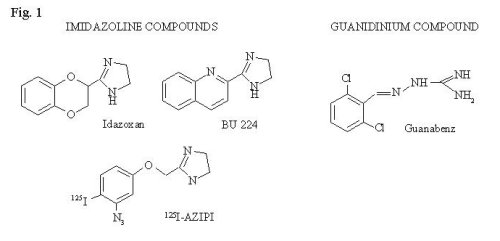 Fig. 1: Chemical structures of several imidazoline or guanidinium compounds.
Fig. 1: Chemical structures of several imidazoline or guanidinium compounds.
Several imidazoline and guanidinium compounds have been reported to inhibit MAO-B activity, however at concentrations one to two orders of magnitude higher than those that would likely saturate the IBD (Tesson et al., 1995, Carpene, C. et al., 1995, Palaty et al., 1989).
To investigate the relationship between inhibition of enzyme activity and interaction of compounds with the IBD on MAO-B we compared the potency for enzyme inhibition to the potency for displacement of radiolabeled imidazolines. The imidazoline BU224 and the guanidinium guanabenz exhibit Ki values of 1-10 nM for the IBD on MAO-B as determined in radioligand binding studies with 3H-idazoxan or photoaffinity labeling with 125I-AZIPI.
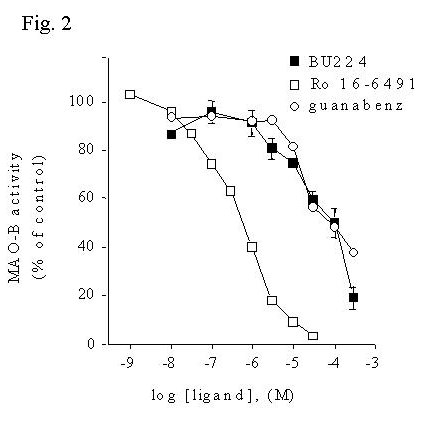 Fig. 2: Imidazoline / guanidinium compounds inhibit activity of MAO-B in human liver membranes.
Fig. 2: Imidazoline / guanidinium compounds inhibit activity of MAO-B in human liver membranes.
Fig. 2 MAO-B activity was measured in human liver membranes using [14C]phenylethylamine as substrate in the presence or absence of increasing concentrations of inhibitors. Data are presented as mean ± S.E.M. of three experiments in duplicate.
Although these results indicate that certain imidazoline / guanidinium compounds indeed inhibit MAO-B activity, the concentrations necessary (IC50's > 10 mM) are much higher than the concentrations of these compounds that bind the IBD of MAO-B ( Ki's 1 - 10 nM). This raised the question of whether the inhibition of MAO-B could be occurring by a secondary interaction of these compounds with the enzyme active site. Displacement of binding of the mechanism-based MAO-B inhibitor [3H]Ro 19-6327 by BU224 and guanabenz was determined and compared to the inhibition of MAO-B activity.
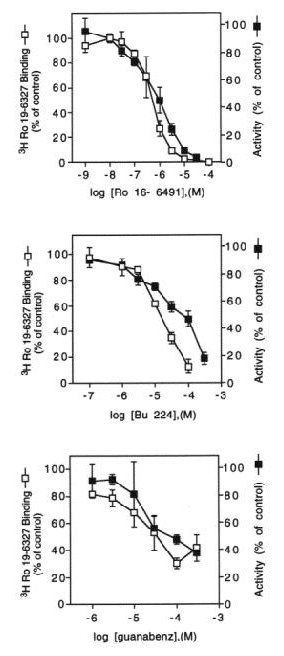 Fig. 3: Inhibition of MAO-B by BU224 and guanabenz occurs at concentrations that displace binding of [3H]Ro 19-6327.
Fig. 3: Inhibition of MAO-B by BU224 and guanabenz occurs at concentrations that displace binding of [3H]Ro 19-6327.
Fig. 3 MAO-B activity or binding of the radiolabeled enzyme inhibitor, [3H]Ro 19-6327, was measured in liver membranes following incubation with increasing concentrations of Ro 16-6491, BU224 or guanabenz. The data are expressed as percent of specific binding (7808 ± 2155 dpm) or enzyme activity (121 ± 5.4 pmol product / 20 min) in the absence of inhibitors and represent the mean ± S.E.M. of 2-3 experiements.
The concentrations of imidazoline or guanidinium compounds necessary to inhibit MAO-B activity are sufficient to displace binding of the enzyme inhibitor [3H]Ro 19-6327, suggesting that enzyme inhibition is due to a secondary interaction of these compounds with the enzyme active site.
Previous studies using the photoaffinity probe, [125I]-AZIPI, indicated that platelet membranes contain few high affinity I2 binding sites relative to the amount of MAO-B present (Raddatz et al., 1995). These observations indicate that the IBD is either not accessible or is not present on all platelet MAO-B. To further evaluate the functional role of the K149-M222 binding domain, the effects of these compounds on platelet MAO-B activity was determined.
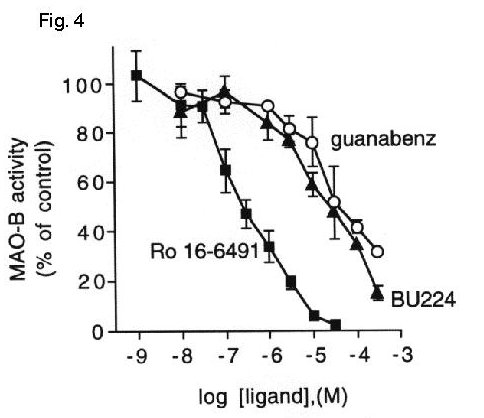 Fig. 4: Imidazoline / guanidinium compounds inhibit platelet MAO-B activity.
Fig. 4: Imidazoline / guanidinium compounds inhibit platelet MAO-B activity.
Fig. 4 MAO-B activity was measured in platelet membranes in the absence or presence of increasing concentrations of Ro 16-6491, BU224 or guanabenz. Data are expressed as percent activity in the absence of inhibitors (132 ± 12 pmol product / 20 min) and represent the mean ± S.E.M. for 2-3 experiments performed in duplicate.
Both BU224 and guanabenz inhibited MAO-B activity equally well in liver (IC50= 62 mM and 64 mM, respectively) and platelet membranes (IC50= 25 mM and 50 mM, respectively) indicating that the lack of accessibility of the IBD on platelet MAO-B had no effect on the enzyme inhibition by these compounds.
To further investigate the question of the lack of high affinity imidazoline/guanidinium binding on certain populations of MAO-B, the accessibility of the IBD on MAO-B was compared in a variety of tissues. First, aliquots of membrane protein from human liver, adipocytes, kidney, brain and platelets containing roughly equivalent amounts of MAO-B were determined by immunoblotting and by binding of the mechanism-based MAO inhibitor, [3H]-pargyline.
Then, the IBD on MAO-B was identified using the I2-selective photoaffinity probe, [125I]-AZIPI.
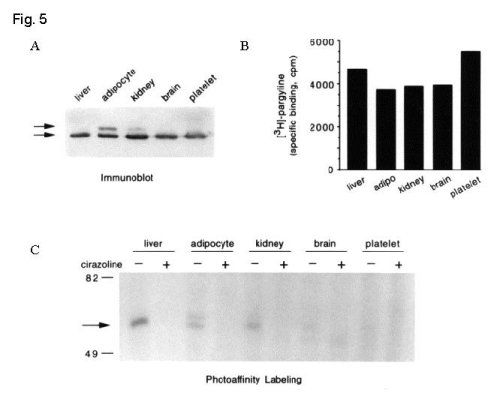 Fig. 5: Tissue-dependent accessibility of the IBD on MAO-B.
Fig. 5: Tissue-dependent accessibility of the IBD on MAO-B.
Fig. 5 A. Membrane protein from human liver (75 µg), adipocytes (50 µg), kidney (100 µg), brain (125 µg) and platelets (400 µg) were separated by SDS-PAGE . MAO-A (Mr ~ 63,000) and MAO-B (Mr ~ 55,000) were identified by immunoblotting of gel transfers. B. Identical amounts of each membrane as in (A) were incubated with 100 nM [3H]-pargyline in the presence of 0.1 mM clorgyline to prevent interaction with MAO-A. Non-specific binding (2 -10 % of total binding) was determined in the presence of 100 mM pargyline and data on the graph represent the average of two experiments performed in duplicate. C. Aliquots of membrane (2-fold the protein as in A) were photolabeled with 2 nM [125I]-AZIPI in the absence and presence of 100 µM cirazoline and separated by SDS-PAGE. The arrow indicates the migration of proteins with Mr ~ 55,000 similar to that of human MAO-B. Data are representative of two expe riments.
These results demonstrate that accessibility of the IBD on MAO-B is tissue-dependent (liver > adipocyte ~ kidney > brain > platelet). The source and significance of this heterogeneity within MAO-B molecules remains unclear.
Discussion and Conclusion
The high affinity interaction of selected imidazoline/ guanidinium compounds with MAO-A and B suggests that some pharmacological effects of these compounds may involve altered levels of monoamine neurotransmitters via regulation of enzyme activity.
Indeed, central administration of imidazolines selective for I2 imidazoline binding proteins resulted in increased levels of neurotransmitter metabolites in the central nervous system and the metabolic profile was consistent with MAO inhibition (Jordan et al., 1996). It is also of note that the imidazoline idazoxan is effective in the management of bipolar affective disorder, a disease that is also treated with MAO inhibitors. However, it is difficult to explain some of the functional actions of these compounds based on a modification of monoamine oxidase enzymatic activity. This observation would suggest that there are additional imidazoline binding sites involved in the action of these compounds or that perhaps MAO-B subserves significant physiological functi ons unrelated to inhibition of monoamine neurotransmitter metabolism (Berry et al., 1994)).
The inhibition of MAO-B by imidazoline ligands observed in vitro in human liver membranes does not involve the high affinity IBD from K149-M222, but rather involves the enzyme site identified by the reversible enzyme inhibitor Ro 19-6327 based on the following points.
1) the concentrations that inhibit MAO-B activity in vitro are one to two orders of magnitude greater than those necessary to saturate the imidazoline binding domain (IBD) on MAO-B and are sufficient to displace binding of the enzyme inhibitor [3H]Ro 19-6327.
2) the imidazoline BU224 and the guanidinium compound guanabenz inhibit MAO-B activity in both liver and platelet even though the IBD on platelet MAO-B is not accessible for binding of these compounds. These results suggest that the in vitro inhibition of MAO activity demonstrated by imidazoline / guanidinium compounds results from secondary interactions with the enzyme active site. MAO-B from the different tissues studied is recognized by selectiv e antibodies, binds 3H-pargyline and oxidizes substrates as expected.
However, MAO-B from the different tissues is not equally able to recognize the imidazoline [125I]-AZIPI. This tissue-selective accessibility of the IBD on MAO-B identifies a previously undescribed heterogeneity within MAO-B molecules. It is unclear whether the different populations of MAO-B are defined by post-translational modification of the protein, the involvement of oligomeric enzyme complexes, tight association of endogenous substances or whether the two populations of enzyme are segregated to different mitochondria within the same cell. Multiple types of MAO-B could perhaps also be generated as splice variants differing in the enzyme domain that recognizes imidazoline/guanidinium ligands. A full understanding of the functional consequences of occupation of the IBD on enzyme activity will eventually require isolation of the subpopulation of MAO-B that contains an accessible domain for ligands of this chemi cal class.
References
- Atlas, D. and Y. Burstein. Eur. J. Biochem.144:287-293
- (1984)Berry, M.D., A.V. Juorio and I.A. Patterson. Prog. in Neurobiol. 42:375-391 (1994)
- Carpene, C., P. Collon, A. Remaury, A. Cordi, A. Hudson, D. Nutt and M. Lafontan. J. Pharmacol. Exp. Ther. 272:681-688 (1995)
- Cesura A.M., J. Gottowik, H.-W. Lahm, G. Lang, R. Imhof, P. Malherbe, U. Röthlisberger and M. Prada. Eur. J. Biochem. 236:996-1002 (1996).
- Jordan, S., H.C. Jackson, D.J. Nutt and S.L. Handley. J. Psychopharmacol. 10:273-278 (1996)
- Lanier, S.M., B. Ivkovic, I. Singh, J.L. Neumeyer and V. Bakthavachalam. J. Biol. Chem. 268:16047-16051 (1993).
- Palaty, V. and E.J. Cragoe Jr.. Mol. Pharmacol. 36:296-301 (1989)
- Raddatz, R., A. Parini and S.M. Lanier. J. Biol. Chem. 270:27961-27968 (1995)
- Raddatz, R., A. Parini amd S.M. Lanier. Mol. Pharmacol. 52:549-553 (1997)
- Tesson, F., I. Limon-Boulez, P. Urban, M. Puype, J. Vandekerckhove, I. Coupry, D. Pompon and A. Parini. J. Biol. Chem.270:9856-9861 (1995)
| Discussion Board | Previous Page | Your Symposium |