Invited Symposium: Medicinal Plants and Drug Actions
| INABIS '98 Home Page | Your Session | Symposia & Poster Sessions | Plenary Sessions | Exhibitors' Foyer | Personal Itinerary | New Search |
Results
Effects of TET on Ca2+ release and Ca2+ entry caused by bembesin and TG:
It is reported that TET alone increases [Ca2+]i in rat alveolar type II cells (18) and human leukaemic HL-60 cells (10). However, the addition of TET (10 and 100 microM) before bombesin or TG did not affect the resting level of [Ca2+]i in glioma C6 cells (Fig. 1). Furthermore, a higher concentration (300 microM) of TET did not increase [Ca2+]i (data not shown).
Bombesin (100 nM) and TG (100 nM) caused a rapid elevation of [Ca2+]i after which it decreased to a sustained level in [Ca2+]i (Fig. 1). TET inhibited both the sustained and peak responses of [Ca2+]i to bombesin and TG in a dose-dependent manner. To further investigate the effects of TET on Ca2+ entry, we cumulatively added TET after the sustained level in [Ca2+]i induced by bombesin and TG (Fig. 2). The sustained increase in the level of [Ca2+]i evoked by bombesin and TG was inhibited by TET in a dose-dependent manner. Thirty and 100 microM TET abolished Ca2+ entry induced by bombesin and TG, respectively.
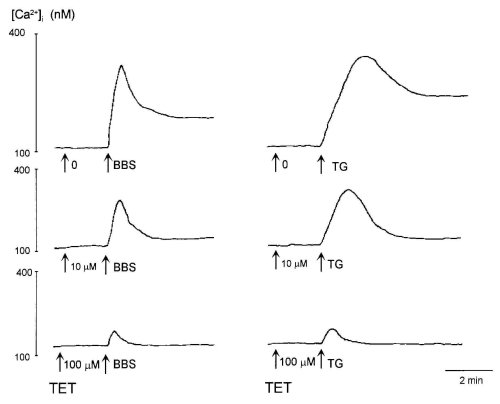 Figure 1: Effects of tetrandrine (TET) on [Ca2+]i evoked by 100 nM bombesin (BBS, left traces) and 100 nM thapsigargin (TG,
right traces).
Figure 1: Effects of tetrandrine (TET) on [Ca2+]i evoked by 100 nM bombesin (BBS, left traces) and 100 nM thapsigargin (TG,
right traces).
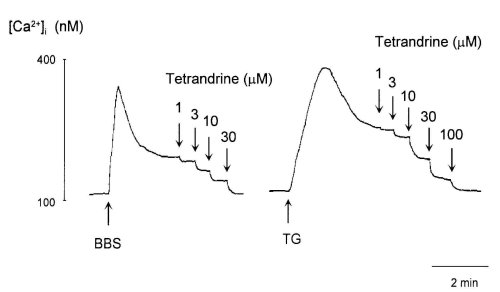 Figure 2: Effects of tetrandrine on the sustained elevation of [Ca2+]i induced by 100 nM bombesin (BBS, left trace) and 100
nM thapsigargin (TG, right trace).
Figure 2: Effects of tetrandrine on the sustained elevation of [Ca2+]i induced by 100 nM bombesin (BBS, left trace) and 100
nM thapsigargin (TG, right trace).
Figure 3 shows the dose-response curves of peak and sustained elevations of [Ca2+]i induced by bombesin and TG for TET, respectively. The doses of TET needed to abolish the sustained and peak increases in [Ca2+]i induced by bombesin and TG were 30 microM and 300 microM, respectively. These results suggested that TET inhibited Ca2+ entry as well as Ca2+ release evoked by bombesin and TG.
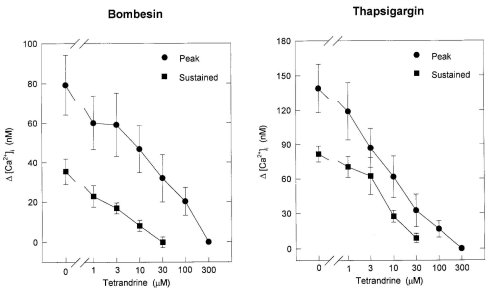 Figure 3: Dose-response curves of the peak and sustained elevation of [Ca2+]i evoked by bombesin (left) and thapsigargin
(right) for tetrandrine. The ordinate expresses as the net increase in [Ca2+]i ( [Ca2+]i) determined by subtracting the
basal level of [Ca2+]i from the peak or the level of sustained elevation of [Ca2+]i. Each point is the mean .$B!^.(B S.E. of
3-6 separate experiments.
Figure 3: Dose-response curves of the peak and sustained elevation of [Ca2+]i evoked by bombesin (left) and thapsigargin
(right) for tetrandrine. The ordinate expresses as the net increase in [Ca2+]i ( [Ca2+]i) determined by subtracting the
basal level of [Ca2+]i from the peak or the level of sustained elevation of [Ca2+]i. Each point is the mean .$B!^.(B S.E. of
3-6 separate experiments.
Effects of TET on IP3 accumulation:
Bombesin (100 nM) induced an about 8-fold increase of IP3 accumulation from the control level (Fig. 4). TG (100 nM) and TET (100 microM) did not increase IP3 formation by themselves. TET markedly inhibited IP3 accumulation caused by bombesin. This suggested that the inhibitory effects of TET on Ca2+ mobilization evoked by bombesin were partially due to the suppression of IP3 formation.
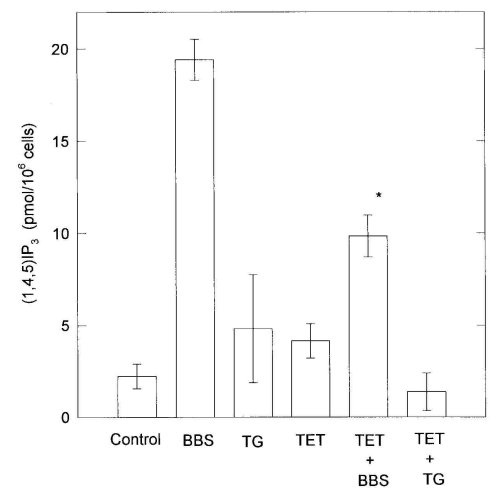 Figure 4: Effects of 100 M tetrandrine (TET) and 100 M hernandezine (HER) on IP3 formation evoked by 100 nM bombesin
(BBS). C6 cells were washed twice with fresh medium and preincubated with medium containing 10 mM LiCl for 10 min at 37
C. The mixture after addition of drugs was further incubated for 30 s. When combined BBS with TET or HER, TET or HER was
added 2 min before the addition of BBS. Each bar is the mean .$B!^.(B S.E. of 3-6 separate experiments. Differences from
corresponding values treated with BBS are denoted by *P<0.01.
Figure 4: Effects of 100 M tetrandrine (TET) and 100 M hernandezine (HER) on IP3 formation evoked by 100 nM bombesin
(BBS). C6 cells were washed twice with fresh medium and preincubated with medium containing 10 mM LiCl for 10 min at 37
C. The mixture after addition of drugs was further incubated for 30 s. When combined BBS with TET or HER, TET or HER was
added 2 min before the addition of BBS. Each bar is the mean .$B!^.(B S.E. of 3-6 separate experiments. Differences from
corresponding values treated with BBS are denoted by *P<0.01.
Effects of TET on Ca2+ release caused by IP3 and TG from permeabilized C6 cells:
Because TET inhibited the peak responses of [Ca2+]i induced by bombesin and TG (Figs. 1 and 3), we examined whether TET inhibited Ca2+ release from intracellular stores evoked by TG. As shown in Fig. 5, IP3 (10 microM) immediately caused release of Ca2+, after which there was a gradual decline to the resting level of Ca2+ release, and TG (100 nM) gradually induced release of Ca2+ from intracellular Ca2+ stores. TET (300 microM) did not affect Ca2+ release by themselves but abolished Ca2+ release from intracellular pools induced by IP3 and TG.
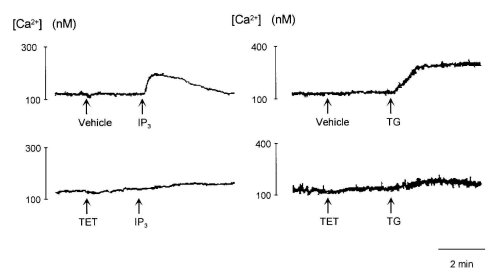 Figure 5:Effects of 300 M tetrandrine (TET) and 300 M hernandezine (HER) on Ca2+ release evoked by 10 M IP3 (left
traces) and 100 nM thapsigargin (TG, right traces) from permeabilized cells.
Figure 5:Effects of 300 M tetrandrine (TET) and 300 M hernandezine (HER) on Ca2+ release evoked by 10 M IP3 (left
traces) and 100 nM thapsigargin (TG, right traces) from permeabilized cells.
Effects of TET on leakage of Ca2+ from extracellular medium into cells:
In the absence of extracellular Ca2+, the addition of 3 mM Ca2+ to extracellular medium slightly increased [Ca2+]i, which indicated Ca2+ entry due to leakage of Ca2+ at the plasma membrane but not that due to entry through Ca2+ channels. TET did not affect this leakage entry of Ca2+ (Fig. 6). On the other hand, after pretreatment with bombesin (100 nM) and TG (100 nM) in the absence of extracellular Ca2+, the addition of Ca2+ caused a rapid elevation of [Ca2+]i followed by a sustained elevation, which indicated Ca2+ entry evoked by drugs (25). TET abolished Ca2+ entry induced by bombesin and TG, suggesting that TET selectively inhibited ROC. Furthermore, these results confirmed those presented in Fig. 2.
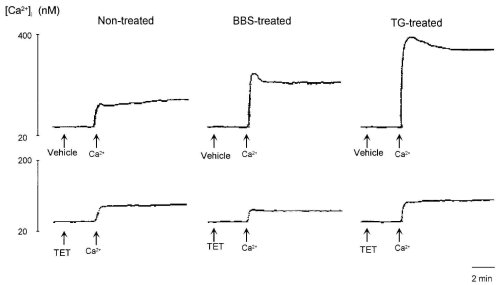 Figure 6: Effects of 100 M tetrandrine (TET) and 100 M hernandezine (HER) on leakage entry of Ca2+ from extracellular
medium and Ca2+ entry evoked by 100 nM bombesin and 100 nM thapsigargin (TG).
Figure 6: Effects of 100 M tetrandrine (TET) and 100 M hernandezine (HER) on leakage entry of Ca2+ from extracellular
medium and Ca2+ entry evoked by 100 nM bombesin and 100 nM thapsigargin (TG).
| <= Materials & Methods | RESULTS | Discussion & Conclussions => |
| Discussion Board | Next Page | Your Symposium |