Invited Symposium: Quinones and Other Reactive Oxygen Species in Neurobiologic, Apoptotic, and Neurotoxic Processes
| INABIS '98 Home Page | Your Session | Symposia & Poster Sessions | Plenary Sessions | Exhibitors' Foyer | Personal Itinerary | New Search |
Introduction
The oxidative pathway of dopamine metabolism in the human brain leads to the formation and accumulation of neuromelanin in the cytoplasm of most nigrostriatal dopaminergic neurons [1-4]. The biosynthesis, structure and physiological functions of neuromelanin in the human substantia nigra are not well understood so far. It is generally accepted that the pigment synthesis starts from the oxidation of dopamine (DA) to its o-quinone, which easily undergoes intramolecular cyclization and further oxidation to dopaminochrome. The rearrangement of dopaminochrome to 5,6-dihydroksyindole, and subsequently oxidative polymerization of the latter compound lead to the formation of indolic eumelanin. In vitro studies have demonstrated that DA o-quinone reacts rapidly with L-cysteine to give cysteinyl conjugates, among these 5-S-cysteinyldopamine (5-S-CysDA) is the major product [5,6,7]. 5-S-CysDA is more easily oxidized than DA to form benzothiazine derivatives [4,8,9]. Such reactions are believed to occur under physiological conditions because 5-S-CysDA has been detected in certain dopaminergic regions of brain (including substantia nigra) and in cerebrospinal fluid of normal human subjects [10,11,12]. Moreover, degradative studies of neuromelanin isolated from human substantia nigra have indicated a significant incorporation of 5-S-CysDA-derived units into the polymer [4,13]. DA o-quinone is also efficiently conjugated with reduced glutathione to form 5-S-glutathionyldopamine [6,8]. Although this conjugate has not been detected in the brain, it is postulated that 5-S-CysDA may result from hydrolysis of 5-S-glutathionyldopamine by intraneuronal peptidase enzymes [10,14].
It is not clear whether the conversion of dopamine into neuromelanin in vivo requires an enzyme contribution. Peroxidase [15]. monoamine oxidase [16], lipoxygenase [17] and prostaglandin H synthetase [18,19] have been suggested to be involved in neuromelanin biosynthesis. On the other hand, it has been postulated that neuromelanin formation is an autoxidative process mediated by reactive oxygen species [1,4,11,20]. In this context, neuromelanin deposition in dopaminergic neurons of the substantia nigra may be regarded as a protective mechanism against potentially cytotoxic o-quinones produced during dopamine oxidation [21,22].
Heavily melanized dopaminergic neurons of the nigrostriatal pathway are known to be particularly susceptible to oxidative stress and degeneration in Parkinson's disease [23-27]. The contribution of neuromelanin to the pathogenesis of Parkinson's disease is still an open question (for review see [28]). In vitro studies have demonstrated that the pigment could act either as an antioxidant [29,30,31] or as a peroxidation-stimulating factor [32,33].
Synthetic dopamine-melanin is commonly used as a model of neuromelanin. However, some data strongly suggest that the pigment of the human substantia nigra consist of indole-type and benzothiazine-type units originated from the oxidation of DA and 5-S-CysDA [4, 13,34]. Therefore, the aim of the presented work was to investigate and compare the effect of synthetic neuromelanins, obtained from DA, 5-S-CysDA or from equimolar mixture of these precursors, on Fe(II)/ascorbate-induced lipid peroxidation.

Materials and Methods
Chemicals
L-cysteine, dopamine (hydrochloride), mushroom tyrosinase (4,200 units/mg of solid), egg yolk lecithin (L-alfa-phosphatidylcholine, type XVI-E, 99% by TLC), linoleic acid (99% by capillary GC), soybean lipoxidase type I-S (51,000 units/mg), 13(S)-hydroxy-(9Z,11E)-octadecadienoic acid (13-HODE), ferrous ammonium sulfate, L-ascorbic acid and 2-thiobarbituric acid (TBA) were obtained from Sigma Chemical Co. (St. Louis,MO). Type I deionized water (Barnstead, NANOpure, bioresearch grade) and solvents of HPLC grade (Sigma-Aldrich) were used in all experiments.
Preparation of 5-S-CysDA and model neuromelanins
5-S-CysDA was obtained by tyrosinase-catalyzed oxidation of dopamine in the presence of cysteine, and purified by ion-exchange chromatography (Dowex 50Wx2) according to Ito et al. [6]. Model neuromelanins were prepared by oxidative polymerization of dopamine (DA-melanin), 5-S-cysteinyldopamine (CysDA-melanin) and equimolar mixture of both precursors (DA/CysDA-melanin). Solutions of the melanin precursors (5 mM) in Tris-HCl buffer (50 mM, pH 7.4) were aerated for 72 hours at room temperature. The insoluble melanins formed during such oxidation were separated by centrifugation, washed three times with water and stored as aqueous suspensions of known concentrations. Elemental analysis of the model neuromelanins was performed by the Center for Molecular and Macromolecular Research of the Polish Academy Sciences in Lodz.
Measurements of lipid peroxidation
Linoleic acid micelles and lecithin liposomes were used as model lipid systems to determine the effect of synthetic neuromelanins on lipid peroxidation. Multilamellar liposomes were freshly prepared as described by Bangham et al. [35]. A solution of egg lecithin in chloroform was evaporated under argon, and the resulting dry lipid film was dispersed in 50 mM Tris-HCl buffer, pH 7.4. Lipid peroxidation was induced by adding ferrous ions and ascorbic acid (final concentrations 20mM and 100mM, respectively) to the suspensions containing linoleic acid (1.5 mM) or liposomal lecithin (3.5 mg/ml) and various concentrations of melanins in Tris-HCl buffer (50 mM, pH 7.4). Incubations were carried out for 24 h at 37oC in a water bath with gently shaking. The accumulation of lipid peroxidation products was assayed by the thiobarbituric acid (TBA) test [36]. The absorbance of TBA-reactive substances (TBARS) was measured at 532 nm before and after incubation. The extent of lipid peroxidation was expressed as a percent of the control incubated without melanin.
Incubation of 13-HPODE with melanins
13-Hydroperoxy-9,11-octadecadienoic acid (13-HPODE) was obtained by enzymatic oxidation of linoleic acid with soybean lipoxidase, as described previously [31]. The concentration of HPODE was quantified at 234 nm using the molar absorption coefficient of 23,000 M-1 cm-1 [37]. 13-HPODE (100 mmg/ml) in Tris- HCl buffer (50 mM, pH 7.4) at 37oC with gently shaking. After 24 h each sample was filtered (filter Millex GV13, pore diameter 0,22 mm; Millipore) to remove melanin and products of the incubation were analyzed by HPLC.
HPLC analysis
Analytical reverse-phase HPLC was performed with a Hewlett-Packard model 1050 liquid chromatograph equipped with a HP 1100 diode array detector and interfaced to HPLC ChemStation (HP). The incubation products were separated on the Eurospher 100 C18 column (particle size 5mm, 250x4mm; Saulentechnik Knauer) eluted isocratically with acetonitryle:water:phosphoric acid (55:45:0.1) at a flow rate of 1 ml/min at 30oC. The eluent was monitored at 234 nm. Linoleic acid was separated on the same column and eluted using acetonitryle:water:phosphoric acid (90:10:0.1), and detected at 206 nm (Rt=7.5 min).Under the chromatographic condition used 13-HPODE and 13-HODE were eluted at 23.6 and 21.1 min, respectively. These compounds were identified by matching their retention times and UV spectra with authentic standards. Quantification was made by reference to calibration curves.

Results
Elemental analysis showed that model neuromelanins obtained from CysDA and from DA/CysDA mixture contained 10.03±0.12% and 6.19±0.18% sulfur, and their sulfur/nitrogen ratios were 1.03 and 0.65 respectively. These data indicate that CysDA-derived units have been indeed incorporated into the two polymers in different proportions.
Study of the effect of model neuromelanins on Fe(II)/ascorbate-induced peroxidation of linoleic acid demonstrated that all melanins tested significantly decreased the yield of TBARS compared to the control, as can be seen in Figure 1.
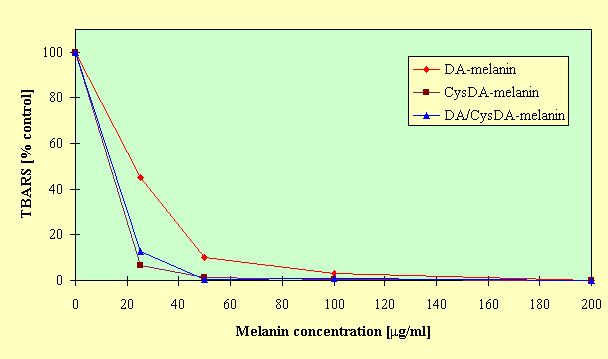
Fig.1. Effect of model neuromelanins on Fe(II)/ascorbate - induced peroxidation of linoleic acid. The reaction mixture contained linoleic acid (1.5 mM), melanin at indicated concentration, ascorbic acid (100
mM), ferrous ammonium sulfate (20 mM) and Tris-HCl buffer (50 mM, pH 7.4) in a total volume of 10 ml. Peroxidation was initiated by addition of ferrous ions and incubation was carried out at 37oC for 24 h. The extend of peroxidation was assayed by measurement of 2-thiobarbituric acid reactive substances (TBARS) accumulation and expressed as percent of the control incubated without melanin. Results are the mean of triplicate determinations from three experiments.At low concentrations (25 and 50
mg/ml), CysDA-melanin and DA/CysDA-melanin were more efficient inhibitors of peroxidation than DA-melanin. However, at higher concentrations of the melanins there was no difference in their antioxidant action, and peroxidation of linoleic acid was suppressed almost completely. The results of TBA-test have been supported by HPLC monitoring of the course of linoleic acid peroxidation. It was found that the model neuromelanins at concentration of 200 mg/ml prevented the decline of linoleic acid and the formation of its hydroperoxides.The effect of synthetic neuromelanins on lecithin peroxidation in liposome membranes depended on the type and concentration of melanin polymers, as can be seen in Figure 2.
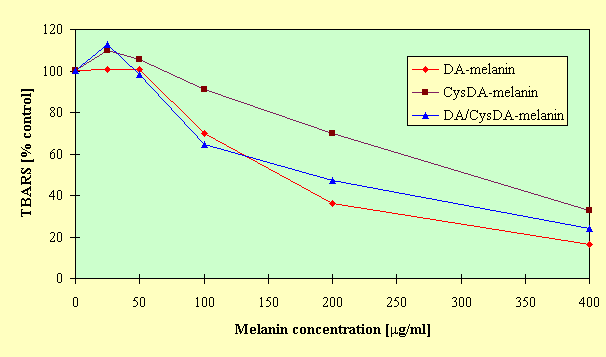
Fig.2. Effect of model neuromelanins on Fe(II)/ascorbate - induced lecithin peroxidation. Experimental conditions as in Fig.1.; linoleic acid was replaced by lecithin (3.5mg/ml).
At low melanin content in the incubation medium (25
mg/ml), CysDA-melanin and DA/CysDA-melanin increased the levels of TBARS to 109.7% and 112.6% of the control respectively (p < 0.05). Such prooxidant effect was not found for DA-melanin. At concentration of 100 mg/ml all the analyzed melanins inhibited lecithin peroxidation, and this inhibitory effect was enhanced with the further increase in melanin concentrations. Antioxidant action of CysDA-melanin was lower than of DA- and DA/CysDA-melanins.To obtain insight into mechanisms of antioxidative action of model neuromelanins, the effect of the polymers on the stability of linoleic acid hydroperoxide was investigated. HPLC analysis showed that the addition of melanin into the incubation medium caused a decrease in the amount of recovered 13-HPODE compared to the control, as can be seen in Figure 3.
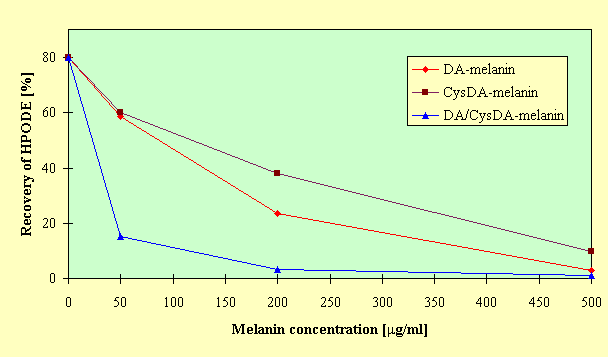
Fig.3. Effect of model neuromelanins on the stability of 13-HPODE. 13-HPODE (100
mM) was incubated without (control) or with melanin in 50 mM Tris-HCl buffer, pH 7.4 at 37oC for 24 h. Recovery of 13-HPODE was determined by HPLC. Initial concentration of 13-HPODE (100 mM) was assumed as 100%. Results are the mean of five experiments.
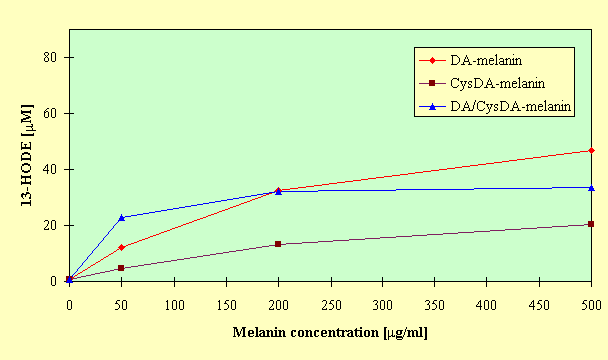
Fig.4. Effect of model neuromelanins on 13-HODE formation. Concentration of 13-HODE formed during incubation of 13-HPODE without (control) or with melanin was determined by HPLC. Each point is the mean of five experiments.
DA/CysDA-melanin accelerated the disappearance of the hydroperoxide more effectively than DA-melanin and CysDA-melanin. The decline of 13-HPODE after treatment with the melanins was accopmanied by the formation of 13-HODE. These results indicate that the model neuromelanins were capable of reducing linoleic acid hydroperoxide to its hydroxyl derivative. The formation of 13-HODE strongly depended on the type and concentration of melanin, as can be seen in Figure 4. The highest amount of 13-HODE formed was found for DA-melanin at concentration of 500
mg/ml. This melanin was the most efficient in the conversion of 13-HPODE to its hydroxyl derivative, while CysDA-melanin was the least effective. At the melanin concentration of 50 mg/ml, 13-HODE was the most effectively formed in a sample with DA/CysDA-melanin. This phenomenon can be explained by the rapid decomposition of 13-HPODE in the presence of this copolymer (Fig.3).
Discussion & Conclusion
Due to the known difficulties to isolate native human neuromelanin, synthetic DA-melanin is commonly used as a model of the natural pigment. Several studies indicate that DA-melanin may affect the process of lipid peroxidation. It has been found that DA-melanin inhibited Fe(II)- or Fe(II)/ascorbate- initiated lipid peroxidation in lecithin and cardiolipin liposomes, and in methyl linoleate aqueous dispersion [29,30,38]. Furthermore, the ability of this melanin to suppress lipid peroxidation induced by UV-light or a water-soluble radical initiator has been demonstrated [30,39]. On the other hand, DA-melanin has been shown to potentiate lipid peroxidation in rat cerebral cortex homogenates after addition of iron [32].
In the presented study, melanin prepared by copolymerization of DA and CysDA, and "pure" phaeomelanin obtained from CysDA were used, in addition to DA-melanin, as models of neuromelanins. DA/CysDA-melanin appears to be the most adequate model of neuromelanin. Degradative studies described by Odh et al. [13] indicate that the pigment of human substantia nigra is a mixed-type melanin and consists of units derived from benzothiazines and from indoles in about equal amounts (the content of phaeomelanin units was in the range 32-60% in different individuals). These authors have also demonstrated that for synthetic melanins formed from CysDA and DA, the sulfur content multiplied by nine gave an estimate of the percentage of benzothiazine-derived units in the polymer. Based on this finding and on sulfur content determined by elemental analysis we estimated the percentage of phaeomelanin units in DA/CysDA-melanin preparation to be about 56%.
The effect of model neuromelanins obtained from CysDA and from DA/CysDA-mixture on Fe(II)/ascorbate-induced lipid peroxidation in linoleic acid micelles and in lecithin liposomes was investigated and compared to the effect of DA-melanin. The results of this study demonstrated that all melanins tested significantly suppressed peroxidation of both linoleic acid and liposomal lecithin in a concentration dependent manner. The inhibitory effect of DA/CysDA-melanin was similar to that of DA-melanin in both lipid systems. This indicates that incorporation of phaeomelanin component into eumelanin polymer does not affect essentially its antioxidant activity. Instead, CysDA-melanin was shown to be less effective inhibitor of lecithin peroxidation than DA-melanin.
Recently, we demonstrated that DA-melanin was capable of reducing linoleic acid hydroperoxide to its more stable hydroxyl derivative, both in the absence and in the presence of ferrous ions [31]. The results of the present study indicate that DA/CysDA-melanin and CysDA-melanin also posses the abilities to reduce the fatty acid hydroperoxide. The effectiveness of CysDA-melanin in conversion of 13-HPODE to 13-HODE was lower than DA-melanin and DA/CysDA-melanin. The reductive inactivation of lipid hydroperoxides is known to prevent hydroperoxide-dependent secondary lipid peroxidation. Our results suggest that the model neuromelanins can act as "chain-breaking" antioxidants.
It is known, that DA-melanin, like other eumelanins, has iron-chelating capability [32,33]. Melanin-bound ferric ions are catalytically inactive and may be released from the complex only by strong reductants. There is some evidence that antioxidant action of DA-melanin is due to its ability to sequester redox-active iron ions [30,33]. Nevertheless, lipid chains are prevented from peroxidative damages as long as melanin capacity to bind iron ions is not exhausted. Zareba et al. [33] have demonstrated that DA-melanin saturated with ferric ions could enhance the formation of free hydroxyl radicals by redox activation of the ions, and postulated that under conditions, that stimulate the release of bound iron, melanin acts as an efficient prooxidant. Such prooxidant effect was observed in our experiments only when the lowest concentrations of DA/CysDA-melanin and CysDA-melanin were investigated.
Our studies have proved that mixed-type melanin formed by copolymerization of dopamine and 5-S-cysteinydopamine, and phaeomelanin originated from cysteinyldopamine are able to inhibit the process of lipid peroxidation induced by ferrous ions in the presence of ascorbic acid. Antioxidant efficiences of DA/CysDA-melanin and DA-melanin were similar, and some higher than of CysDA-melanin. The results obtained strongly suggest, that under physiological conditions neuromelanin can act as natural antioxidant. The fatty acid hydroperoxide-reducing ability demonstrated for the model neuromelanins appears to be involved in the mechanism of antioxidative activity of neuromelanin.

References
1.Rodgers, AD and Curzon, G (1975) Melanin formation by human brain in vitro. Journal of Neurochemistry, 24:1123-1129.
2.Graham, DG (1978) Oxidative pathways for catecholamines in the genesis of neuromelanin and cytotoxic quinones. Molecular Pharmacology, 14:633-643.
3.Marsden, CD (1983) Neuromelanin and Parkinson's disease. Journal of Neural Transmission, Supplementum, 19:121-141.
4.Carstam, R; Brinck, C; Hindemith-Augustsson, A; Rorsman, H and Rosengren, E (1991) The neuromelanin of the human substantia nigra. Biochimica et Biophysica Acta, 1097:152-160.
5.Tse, DCS; McCreery, RL and Adams, RN (1976) Potential oxidative pathways of brain catecholamines. Journal of Medicinal Chemistry, 19:37-40.
6.Ito, S; Fujita, K; Yoshioka, M; Sienko, D and Nagatsu,T (1986) Identyfication of 5-S- and 2-S-cysteinyldopamine and 5-S-glutathionyldopamine formed from dopamine by high-performance liquid chromatography with electrochemical detection. Journal of Chromatography, 375:134-140.
7.Shen, XM and Dryhurst,G (1996) Further insights into the influence of L-cysteine on the oxidation chemistry of dopamine: reaction pathways of potential relevance to Parkinson's disease. Chemical Research in Toxicology, 9:751-763.
8.Zhang, F and Dryhurst, G (1994) Effects of L-cysteine on the oxidation chemistry of dopamine: new reaction pathways of potential relevance to idiopathic Parkinson's disease. Journal of Medicinal Chemistry, 37:1084-109
9.Zhang, F and Dryhurst,G. (1995) Reactions of cysteine and cysteinyl derivatives with dopamine-o-quinone and further insights into the oxidation chemistry of 5-S-cysteinyldopamine: potential relevance to idiopathic Parkinson's disease. Chemical Research in Toxicology, 9:751-763.
10.Rosengren, E; Linder-Eliasson, E and Carlsson, A (1985) Detection of 5-S-cysteinyldopamine in human brain. Journal of Neural Transmission, 63:247-253.
11.Fornstedt ,B; Brun, A; Rosengren, E and Carlsson, A (1989) The apparent autoxidation rate of catechols in dopamine-rich regions of human brains increases with the degree of depigmentation of substantia nigra. Journal of Neural Transmission, 1:279-295.
12.Cheng, FC; Kuo, JS; Chia, LG and Dryhurst, G (1996) Elevated 5-S-cysteinyldopamine/homovanillic acid ratio and reduced homovanilic acid in cerebrospinal fluid: possible markers for and potential insights into the pathoetiology of Parkinson's disease. Journal of Neural Transmission, 103:433-446.
13.Odh, G; Carstam, R; Paulson, J; Wittbjer, A; Rosengren, E and Rorsman, H (1994) Neuromelanin of the human substantia nigra: a mixed- type melanin. Journal of Neurochemistry, 62:2030-2036.
14.Miller, RT; Lau, SS and Monks, TJ (1995) Metabolism of 5-(glutathion-S-yl)-alfa-methyldopamine following intracerebroventricular administration to male Sprague-Dawley rats. Chemical Research in Toxicology, 8:634-641.
15.Okun, MR (1997) The role of peroxidase in neuromelanin synthesis: a review. Physiological Chemistry and Physics & Medical NMR, 29:15-22.
16.Rabey, JM and Hefti, F (1990) Neuromelanin synthesis in rat and human substantia nigra. Journal of Neural Transmission, 2:1-14.
17.Rosei, MA; Blarzino, C; Foppoli, C; Mosca, L and Coccia, R (1994) Lipoxygenase-catalyzed oxidation of catecholamines. Biochemical and Biophysical Research Communications, 200:344-350.
18.Hastings, TG (1995) Enzymatic oxidation of dopamine: the role of prostaglandin H synthetase. Journal of Neurochemistry, 64:919-924.
19.Mattammal, MB; Strong, R; Lakshuri, VM; Chung, HD and Stephenson, AH (1995) Prostaglandin H synthetase-mediated metabolism of dopamine: implication for Parkinson's disease. Journal of Neurochemistry, 64:1645-1654.
20.Enochs, WS; Sarna, T; Zecca, L; Riley, PA and Swartz, HM (1994) The roles of neuromelanin, binding of metal ions, and oxidative cytotoxicity in the pathogenesis of Parkinson's disease: a hypothesis. Journal of Neural Transmission, 7:83-100.
21.Graham, DG (1979) On the origin and significance of neuromelanin. Archives of Pathololgy and. Laboratory Medicine. 103:359-362.
22.Smythies, J (1996) On the function of neuromelanin. Proceedings of the Royal. Society London. B, 263:487-489.
23.Hirsch, E; Graybiel, AM and Agid, YA (1988) Melanized dopaminergic neurons are differentially susceptible to degeneration in Parkinson's disease. Nature, 334:345-348.
24.Kastner, A; Hirsch, EC; Lejeune, O; Javoy-Agid, F; Rascol, O and Agid, Y (1992) Is the vulnerability of neurons in the substantia nigra of patients with Parkinson's disease related to their neuromelanin content? Journal of Neurochemistry, 59:1080-1089.
25.Dexter, DT; Carter, CJ; Wells FR; Javoy-Agid, F; Agid, Y; Lees, AJ; Jenner, P and Marsden, CD (1989) Basal lipid peroxidation in substantia nigra is increased in Parkinson's disease. Journal of Neurochemistry, 52:381-389.
26.Jenner, P; Dexter, DT; Sian, J; Schapira, AHV and Marsden, CD (1992) Oxidative stress as a cause of nigral cell death in Parkinson's disease and incidental Lewy body disease. Annals of Neurology, 32:S82-S87.
27.Fahn, S and Cohen, G (1992) The oxidant stress hypothesis in Parkinson's disease: evidence supporting it. Annals of Neurology, 32:804-812.
28.D'Ischia, M and Prota, G (1997) Biosynthesis, structure, and function of neuromelanin and its relation to Parkinson's disease: a critical update. Pigment Cell Research, 10:370-376.
29.Porebska-Budny, M; Sakina,NL; Stepien, KB; Dontsov, AE and Wilczok, T (1992) Antioxidative activity of synthetic melanins. Cardiolipin liposome model. Biochimica et Biophysica Acta, 1116:11-16.
30.Korytowski, W; Sarna, T and Zareba, M (1995) Antioxidant action of neuromelanin: the mechanism of inhibitory effect on lipid peroxidation. Archives of Biochemistry and Biophysics, 319:142-148.
31.Stepien, K; Zajdel, A; Swierczek, G; Wilczok, A and Wilczok,T (1998) Reduction of 13-hydroperoxy-9,11-octadecadienoic acid by dopamine-melanin. Biochemical and Biophysical Research Communications, 244:781-784.
32.Ben-Shachar,D; Riederer, P and Youdim, MBH (1991) Iron-melanin interaction and lipid peroxidation: implications for Parkinson's disease. Journal of Neurochemistry, 57:1609-1614.
33.Zareba, M; Bober, A; Korytowski, W; Zecca, L and Sarna, T (1995) The effect of a synthetic neuromelanin on yield of free hydroxyl radicals generated in model systems. Biochimica et Biophysica Acta, 1271:343-348.
34.Zecca, L; Shima, T; Stroppolo, A; Goj, C; Battiston, GA; Gerbasi, R; Sarna, T and Swartz, HM (1996) Interaction of neuromelanin and iron in substantia nigra and other areas of human brain. Neuroscience, 73:407-415.
35.Bangham, AD; Hill, MW and Miller, NGA (1974) Preparation and use of liposomes as models of biological membranes. In:Methods in Membrane Biology (ed. Korn, ED), Plenum Press, New York-London, 1:1-68.
36.Buege, J and Aust, SD (1978) Microsomal lipid peroxidation. Methods in Enzymology, 52C:302-310.
37.Gibian,M and Vandenberg, P (1987) Product yield in oxygenation of linoleate by soybean lipoxygenase: the value of the molar extinction coefficient in the spectrophotometric assay. Analytical Biochemistry, 163:343-349.
38.Stepien, K and Wilczok, T (1994) Antioxidant activity of model neuromelanins in the process of lipid peroxidation. Current Topics in Biophysics, 18:135-138.
39.Stepien, K; Porebska-Budny, M; Hollek, AM and Wilczok, T (1992) The inhibiting effect of catecholamine-melanins on UV-induced lecithin peroxidation. Journal of Photochemistry and Photobiology.B:Biology, 15:223-231.

| Discussion Board | Previous Page | Your Symposium |
© 1998 Author(s) Hold Copyright