Invited Symposium: Intracellular Traffic of Organelles
| INABIS '98 Home Page | Your Session | Symposia & Poster Sessions | Plenary Sessions | Exhibitors' Foyer | Personal Itinerary | New Search |
Introduction
Clathrin-coated vesicles (CCV) mediate the endocytosis of plasma membrane proteins and the transport of proteins from the trans-Golgi network (TGN) to the endosomal/ lysosomal system (Schmid, 1997; Le Borgne & Hoflack, 1998). One of the most intriguing examples of CCV formation takes place at the nerve terminal where CCVs are involved in the recycling of synaptic vesicles following a burst of exocytic activity (Heuser & Reese, 1973; Cremona & De Camilli, 1997). Both clathrin and its accessory proteins are concentrated in nerve terminals, and synaptic vesicle proteins appear to be the main cargo of CCVs in the brain (Maycox et al., 1992). The role of clathrin coats in synaptic vesicle endocytosis has recently been corroborated by genetic studies in flies (Gonzales-Gaitan & Jackle, 1997).
In this discussion we will primarily focus on recent insights into the mechanism of clathrin-coated vesicle formation at the synapse which shall serve as a model for clathrin-mediated endocytosis in general.
From Pits To Buds
Nerve terminal CCV are comprised of the heavy and light chains of clathrin, the clathrin-associated protein AP180 and the AP-2 adaptor complex which serves to link the clathrin shell to the membrane. AP-2 is a brick-like heterotetrameric complex of two 100-110 kDa subunits (a and b2) and two smaller subunits of 47 (m2) and 17 (s) kDa, respectively (Figure 1).
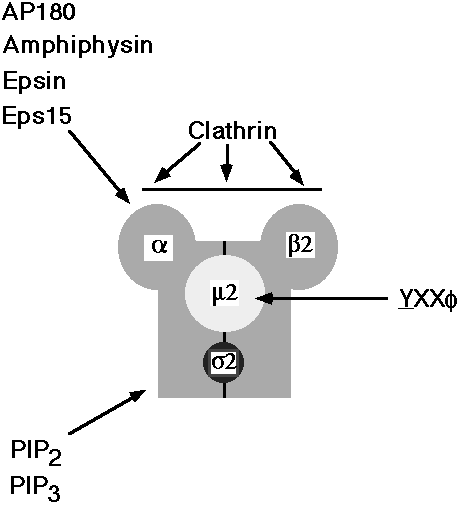 Figure 1: Molecular intercations between clathrin accessory proteins and subunits of AP-2
Schematic diagram illustrating interactions between subunits of the adaptor complex AP-2 and various molecules involved in endocytosis. YXXF represents transmembrane proteins containing tyrosine-based internalization motifs. PIP2, PIP3: phosphatidyl inositol 4,5-bisphosphate and phosphatidyl inositol 3,4,5-trisphosphate, respectively.
Figure 1: Molecular intercations between clathrin accessory proteins and subunits of AP-2
Schematic diagram illustrating interactions between subunits of the adaptor complex AP-2 and various molecules involved in endocytosis. YXXF represents transmembrane proteins containing tyrosine-based internalization motifs. PIP2, PIP3: phosphatidyl inositol 4,5-bisphosphate and phosphatidyl inositol 3,4,5-trisphosphate, respectively.
Clathrin-coated bud formation is initiated by the binding of AP-2 to a putative high affinity docking site of which the synaptic vesicle protein synaptotagmin may be part of (Zhang et al., 1994), followed by the assembly of clathrin triskelia into polyhedral cages (Smith et al., 1998). This process may be aided by AP180, a clathrin-associated assembly protein which also interacts with AP-2 (Wang et al., 1995). Although the nature of the docking site has remained elusive recent evidence suggests that phospholipids such as phosphatidic acid (West et al., 1997) and membrane proteins could cooperate in recruiting adaptors to the plasma membrane (Figure 2).
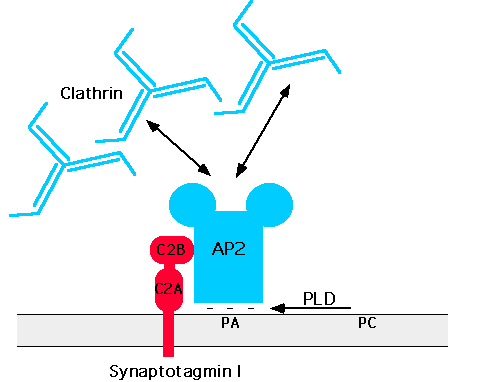 Figure 2: Hypothetical model for the recruitment of clathrin/AP-2 to the plasma membrane
See text for details. C2A, C2B=protein kinase C homologous domains of synaptotagmin; PLD: phospholipase D; PC: phophatidyl choline; PA: phosphatitidic acid.
Figure 2: Hypothetical model for the recruitment of clathrin/AP-2 to the plasma membrane
See text for details. C2A, C2B=protein kinase C homologous domains of synaptotagmin; PLD: phospholipase D; PC: phophatidyl choline; PA: phosphatitidic acid.
This hypothesis is supported by the demonstration that clathrin-coated buds indistinguishable from corresponding structures observed in vivo can assemble on chemically defined protein-free liposomes although assembly requires elevated concentrations of coat proteins (Takei et al., 1998). Similar results have been reported for the budding of COPII (Matsuoka et al., 1998) and COPI (Spang et al., 1998) coated vesicles from synthetic liposomes.
Several accessory proteins which themselves are not an intrinsic part of the coat such as amphiphysins I and II (David et al., 1996, Butler et al., 1997; Leprince et al., 1997; Ramjaun et al., 1997; Wigge et al., 1997), Eps15 (Benmerah et al., 1996; Tebar et al., 1996; Cupers et al., 1998), and epsin (Chen et al.,1998) appear to assist the clathrin/AP-2 coat in the invagination and fission reactions, but their precise function is unclear (Table I).
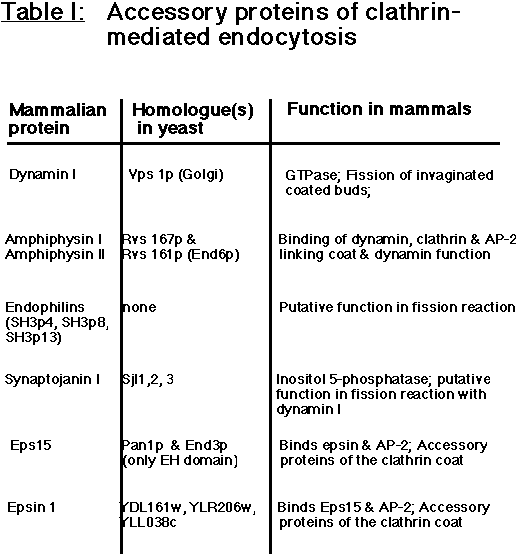 Table I: Accessory proteins of clathrin-mediated endocytosis.
See text for details. Alternative names are given in brackets. In the case of some yeast genes the expression of the encoded ORF has not yet been proven.
Table I: Accessory proteins of clathrin-mediated endocytosis.
See text for details. Alternative names are given in brackets. In the case of some yeast genes the expression of the encoded ORF has not yet been proven.
Several mechanisms might be involved in the temporal and spatial regulation of coated pit nucleation including phosphorylation of adaptors (Wilde and Brodsky, 1996) or clathrin accessory proteins (Bauerfeind et al., 1997; Slepnev et al., 1998), activation of ARF (D' Souza-Schorey et al., 1995; West et al., 1997), local changes in the concentration of phosphoinositides which bind to AP-2 and AP180 (Mc Pherson et al., 1996; Hao et al., 1997; Rapoport et al., 1997) or of other acidic membrane phospholipids (West et al., 1997).
How Clathrin Coated Buds Pinch Off
As first suggested by studies on the shibire mutant of Drosophila, the fission of clathrin-coated vesicles requires the action of the GTPase dynamin which oligomerizes into rings at the neck of the invaginating coated pit (Koenig & Ikeda, 1989; Takei et al., 1995). A number of 'helper' proteins may participate in the dynamin-mediated fission reaction and in its close coupling with the formation of clathrin-coated buds. Recruitment of dynamin to its site of action is mediated by an interaction between a specific sequence in its proline-rich tail and the SH3 domains of amphiphysin I and II (Grabs et al., 1997; Owen et al., 1998). Competitive inhibition of this interaction blocks the endocytosis of synaptic vesicles at the stage of invaginated coated pits (Shupliakov et al.,1997) and inhibits receptor-mediated endocytosis in non-neuronal cells (Wigge et al., 1997) implying that a dynamin-amphiphysin interaction lies at the core of the fission mechanism. Whether dynamin acts as a pinchase (Roos and Kelly, 1997) in this scenario has not yet been firmly proven, but it has been demonstrated that purified dynamin is capable of assembling into oligomeric rings on synthetic liposomes (Sweitzer and Hinshaw, 1998; Takei et al., 1998) and to vesiculate large membrane tubules into small vesicles upon GTP hydolysis (Sweitzer and Hinshaw, 1998). Other important factors facilitating the pinching off of a clathrin-coated vesicle may include local changes in the phospholipid composition at the vesicle neck such as the hydrolysis of phosphoinositides by the inositol 5'-phosphatase synaptojanin I (Mc Pherson et al., 1996). Interestingly, most of the proteins discussed so far have functional homologs in yeast (Geli and Riezman, 1998 for review) suggesting that the basic mechanism of clathrin-mediated endocytosis has been conserved from yeast to neurons (Table I).
Cargo Selection
Two classes of sorting signals have been identified which serve to target transmembrane receptors to clathrin-coated pits: the best defined signal is a tyrosine-containing motif found in TGN38 and the transferrin receptor containing the consensus sequence YXXF, in which F is a large hydrophobic residue. A second signal, NPXY has been first identified in the LDL receptor, but it is not entirely clear whether it is recognized by the same sorting machinery as the tyrosine signal (Kirchhausen et al., 1997; Schmid, 1997). The tyrosine-based motif interacts directly with the m2 chain of AP-2 complexes and this interaction is strongly regulated by phosphoinositides and coat assembly (Rapoport et al., 1997).
Surprisingly, we know very little about the sorting mechanisms responsible for the accumulation of synaptic vesicle (SV) proteins in clathrin-coated pits during their recycling at the synapse. Although some SV proteins contain known internalization signals these signals have not yet been demonstrated to actively contribute to the concentration of these proteins into clathrin-coated pits (Maycox et al., 1992). The only SV protein that has been shown to interact with clathrin and adaptors so far is synaptotagmin. Since synaptotagmin is also implicated in exocytosis, its proposed function as a membrane receptor for clathrin/AP-2 could explain the close coupling of the exo- and endocytic limbs of the synaptic vesicle cycle (Zhang et al., 1994). The use of a SV protein as a nucleation site for clathrin coat assembly would then also serve as a mechanism for cargo selection. Genetic analyses of synaptotagmin mutants in C. elegans seem to support this idea (Jorgensen et al.,1995). An alternative pathway for SV recycling has been proposed which uses the recently identified AP-3 adaptor complex (Faundez et al., 1998). AP-3 appears to be recruited to endosomes by the SV protein synaptobrevin (R. Kelly, personal communication). The relative contributions of these pathways to the recycling of SVs in vivo is unclear at present.
Thus, further studies will be needed to address the question of how precisely the composition of SV membranes is retained or re-established during receycling and which molecular interactions are responsible for this selectivity.
Outlook
Although we have made enourmous progress in establishing the sequence of events leading to the generation of a clathrin-coated vesicle, several important questions remain to be resolved. First, how is coat assembly regulated in response to a variety of stimuli and during the cell cycle? Second, we need to understand the precise mechanism of the dynamin-mediated fission reaction which so far is unique among the known vesicular budding events in its requirement for a pinchase. Third, we will have to better define the function of many of the recently identified accessory and clathrin- associated proteins such as Eps15, epsin, and endophilins. While some of them may lie at the heart of the machinery others may turn out to be specific factors relevant to only some forms of endocytosis or certain states. Fourth, we are only now beginning to unravel the function of membrane lipids in clathrin-coated bud formation and fission. New methods will have to be developed that may allow us to measure local changes in the lipid composition at sites of coated vesicle formation. Lastly, are there isoforms of adaptor complexes, such as the recently identified AP-3 complex (Simpson et al., 1996; Dell Angelica et al., 1997) that can function without the aid of clathrin itself (Dell Angelica et al., 1998) and what is their role in vivo? These new coats may use different mechanisms for membrane targeting and cargo selection although tentative evidence so far speaks against this (Faundez et al., 1998; Ooi et al., 1998). Hopefully, the answers to these and other questions may not lie too far ahead.
References
- Bauerfeind, R., Takei, K., and De Camilli, P. (1997). Amphiphysin I is associated with coated endocytic intermediates and undergoes-stimulation-dependent dephosphorylation in nerve terminals. J. Biol. Chem. 272, 30984-30992.
- Benmerah, A., Begue, B., Dautry-Varsat, A., and Cerf-Bensussan, N. (1996). The ear of alpha-adaptin interacts with the COOH-terminal domain of the Eps 15 protein. J. Biol. Chem. 271, 12111-6.
- Butler, M. H., David, C., Ochoa, G. C., Freyberg, Z., Daniell, L., Grabs, D., Cremona, O., and De Camilli, P. (1997). Amphiphysin II (SH3P9; BIN1), a member of the amphiphysin/Rvs family, is concentrated in the cortical cytomatrix of axon initial segments and nodes of ranvier in brain and around T tubules in skeletal muscle. J Cell Biol 137, 1355-67.
- Chen, H., Fre, S., Slepnev, V. I., Capua, M. R., Takei, K., Butler, M. H., Di Fiore, P. P., and De Camilli, P. (1998). Epsin is an EH-domain-binding protein implicated in clathrin-mediated endocytosis. Nature 394, 793-7.
- Cremona, O., and De Camilli, P. (1997). Synaptic vesicle endocytosis. Curr. Op. Neurobiol. 7, 323-330.
- Cupers, P., Jadhav, A. P., and Kirchhausen, T. (1998). Assembly of clathrin coats disrupts the association between Eps15 and AP-2 adaptors. J Biol Chem 273, 1847-50.
- D'Souza-Schorey, C., Li, G., Colombo, M. I., and Stahl, P. D. (1995). A regulatory role for ARF6 in receptor-mediated endocytosis. Science 267, 1175-1178.
- David, C., McPherson, P. S., Mundigl, O., and De Camilli, P. (1996). A role of amphiphysin in synaptic vesicle endocytosis suggested by its binding to dynamin in nerve terminals. Proc. Natl Acad. Sci. USA 93, 331-335.
- Dell'Angelica, E. C., Klumperman, J., Stoorvogel, W., and Bonifacino, J. S. (1998). Association of the AP-3 adaptor complex with clathrin. Science 280, 431-4.
- Dell'Angelica, E. C., Ohno, H., Ooi, C. E., Rabinovich, E., Roche, K. W., and Bonifacino, J. S. (1997). AP-3: an adaptor-like protein complex with ubiquitous expression. EMBO Journal 16, 917-28.
- Faundez, V., Horng, J. T., and Kelly, R. B. (1998). A function for the AP3 coat complex in synaptic vesicle formation from endosomes. Cell 93, 423-32.
- Geli, M. I., and Riezman, H. (1998). Endocytic internalization in yeast and animal cells: similar and different. J Cell Sci 111, 1031-7.
- Gonzalez-Gaitan, M., and Jackle, H. (1997). Role of Drosophila alpha-adaptin in presynaptic vesicle recycling. Cell 88, 767-76.
- Grabs, D., Slepnev, V. I., Songyang, Z., David, C., Lynch, M., Cantley, L. C., and De Camilli, P. (1997). The SH3 domain of amphiphysin binds the proline-rich domain of dynamin at a single site that defines a new SH3 binding consensus sequence. J. Biol. Chem. 272, 13419-25.
- Hao, W., Tan, Z., Prasad, K., Reddy, K. K., Chen, J., Prestwich, G. D., Falck, J. R., Shears, S. B., and Lafer, E. M. (1997). Regulation of AP-3 function by inositides: Identification of phosphatidylinositol 3,4,5-triphosphate as a potent ligand. J. Biol. Chem. 272, 6393-8.
- Heuser, J. E., and Reese, T. S. (1973). Evidence for recycling of synaptic vesicle membrane during transmitter release at the frog neuromuscular junction. J. Cell Biol. 57, 315-344.
- Jorgensen, E. M., Hartwieg, E., Schuske, K., Nonet, M. L., Jin, Y., and Horvitz, H. R. (1995). Defective recycling of synaptic vesicles in synaptotagmin mutants of Caenorhabditis elegans. Nature 378, 196-9.
- Kirchhausen, T., Bonifacino, J. S., and Riezman, H. (1997). Linking cargo to vesicle formation: receptor tail interactions with coat proteins. Curr Opin Cell Biol 9, 488-95.
- Koenig, J. H., and Ikeda, K. (1989). Disappearance and reformation of synaptic vesicle membrane upon transmitter release observed under reversible blockage of membrane retrieval. J. Neurosci. 9, 3844-3860.
- Le Borgne, R., and Hoflack, B. (1998). Mechanisms of protein sorting and coat assembly: insights from the clathrin-coated vesicle pathway [In Process Citation]. Curr Opin Cell Biol 10, 499-503.
- Leprince, C., Romero, F., Cussac, D., Vayssiere, B., Berger, R., Tavitain, A., and Camonis, J. H. (1997). A new member of the amphiphysin family connecting endocytosis and signal transduction pathways. J. Biol. Chem. 272, 15101-15105.
- Matsuoka, K., Orci, L., Amherdt, M., Bednarek, S. Y., Hamamoto, S., Schekman, R., and Yeung, T. (1998). COPII-coated vesicle formation reconstituted with purified coat proteins and chemically defined liposomes. Cell 93, 263-75.
- Maycox, P. R., Link, E., Reetz, A., Morris, S. A., and Jahn, R. (1992). Clathrin-coated vesicles in nervous tissue are involved primarily in synaptic vesicle recycling. J. Cell Biol. 118, 1379-1388.
- McPherson, P. S., Garcia, E. P., Slepnev, V. I., David, C., Zhang, X. M., Grabs, D., Sossin, W. S., Bauerfeind, R., Nemoto, Y., and De Camilli, P. (1996). A presynaptic inositol-5-phosphatase. Nature 379, 353-357.
- Ooi, C. E., Dell'Angelica, E. C., and Bonifacino, J. S. (1998). ADP-Ribosylation factor 1 (ARF1) regulates recruitment of the AP-3 adaptor complex to membranes. J Cell Biol 142, 391-402.
- Owen, D. J., Wigge, P., Vallis, Y., Moore, J. D., Evans, P. R., and McMahon, H. T. (1998). Crystal structure of the amphiphysin-2 SH3 domain and its role in the prevention of dynamin ring formation EMBO J 17, 5273-85
- Ramjaun, A. R., Micheva, K. D., Bouchelet, I., and McPherson, P. S. (1997). Identification and Characterization of a Nerve Terminal-enriched Amphiphysin Isoform. J. Biol. Chem. 272, 16700-6.
- Rapoport, I., Miyazaki, M., Boll, W., Duckworth, B., Cantley, L. C., Shoelson, S., and Kirchhausen, T. (1997). Regulatory interactions in the recognition of endocytic sorting signals by AP-2 complexes. EMBO J 16, 2240-50.
- Roos, J., and Kelly, R. B. (1997). Is dynamin really a "pinchase"? Trends Cell Biol. 7, 257-259.
- Schmid, S. L. (1997). Clathrin-coated vesicle formation and protein sorting: an integrated process. Annu Rev Biochem 66, 511-48.
- Shupliakov, O., Low, P., Grabs, D., Gad, H., Chen, H., David, C., Takei, K., De Camilli, P., and Brodin, L. (1997). Synaptic vesicle endocytosis impaired by disruption of dynamin-SH3 domain interactions. Science 276, 259-63.
- Simpson, F., Bright, N. A., West, M. A., Newman, L. S., Darnell, R. B., and Robinson, M. S. (1996). A novel adaptor-related protein complex. J. Cell Biol. 133, 749-760.
- Slepnev, V. I., Ochoa, G. C., Butler, M. H., Grabs, D., and Camilli, P. D. (1998). Role of phosphorylation in regulation of the assembly of endocytic coat complexes. Science 281, 821-4.
- Smith, C. J., Grigorieff, N., and Pearse, B. M. (1998). Clathrin coats at 21 A resolution: a cellular assembly designed to recycle multiple membrane receptors [In Process Citation]. EMBO J 17, 4943-53.
- Spang, A., Matsuoka, K., Hamamoto, S., Schekman, R., and Orci, L. (1998). Coatomer, arf1p, and nucleotide are required to bud coat protein complex I-coated vesicles from large synthetic liposomes [In Process Citation]. Proc Natl Acad Sci U S A 95, 11199-204.
- Takei, K., Haucke, V., Slepnev, V., Farsad, K., Salazar, M., Chen, H., and De Camilli, P. (1998). Generation of coated intermediates of clathrin-mediated endocytosis on protein-free liposomes. Cell 94, 131-41.
- Tebar, F., Sorkina, T., Sorkin, A., Ericsson, M., and Kirchhausen, T. (1996). Eps15 is a component of clathrin-coated pits and vesicles and is located at the rim of coated pits. J. Biol. Chem. 271, 28727-28730.
- Wang, L. H., Südhof, T. C., and Anderson, R. G. W. (1995). The appendage domain of alpha-adaptin is a high affinity binding site for dynamin. J. Biol. Chem. 270, 10079-10083.
- West, M. A., Bright, N. A., and Robinson, M. S. (1997). The role of ADP-ribosylation factor and phospholipase D in adaptor recruitment. J. Cell Biol. 138, 1239-1254.
- Wigge, P., Kohler, K., Vallis, Y., Doyle, C. A., Owen, D., Hunt, S. P., and McMahon, H. T. (1997). Amphiphysin heterodimers: potential role in clathrin-mediated endocytosis. Mol Biol Cell 8, 2003-15.
- Wilde, A., and Brodsky, F. M. (1996). In vivo phosphorylation of adaptors regulates their interaction with clathrin. J. Cell Biol. 135, 635-645.
- Zhang, J. Z., Davletov, B. A., Sudhof, T. C., and Anderson, R. G. (1994). Synaptotagmin I is a high affinity receptor for clathrin AP-2: implications for membrane recycling. Cell 78, 751-760.
| Discussion Board | Previous Page | Your Symposium |