Immunology & Immunological Disorders Poster Session
| INABIS '98 Home Page | Your Session | Symposia & Poster Sessions | Plenary Sessions | Exhibitors' Foyer | Personal Itinerary | New Search |
Introduction
The main immunoglobulin fraction of fowls is called IgY, in order to distinguish it from the mammalian IgG. There are some crucial points of difference between IgG and IgY (7) as can be seen in Figure 1.
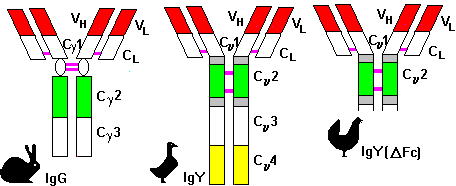 Fig. 1: The structural differences between mammalian IgG and avian IgY.
Fig. 1: The structural differences between mammalian IgG and avian IgY.
Avian IgY is more resistant to temperature, pH and ion strength of environment than IgG. IgY has no reactivity to mammalian auto-antibodies, Fc-receptors or RBCs. In mammalian systems, these cross-reactions can be eliminated using avian-originated antibodies (ABs). Serum IgY is identical to yolk AB, as has been proven in comparative studies. The isolation and purification for further different uses of AB has great potential.
The importance of eggs as a source of specific antibodies is well known. Egg yolk contains 8-20 mg of immunoglobulin (IgY) per ml. However, the major problem in isolation is the removal of lipids which are present in high concentrations. Several methods had been developed by employing water dilution and solvent extraction to separate yolk plasma proteins from the granules and lipids.
Taking into consideration the discussed methods, a combined (water dilution and dextran sulphate precipitation, WD+DS) technique was used in our experiments.
Materials and Methods
Chemicals and equipment
Chemicals used: dextran sulphate (ICN - USA), salts of buffers (Reanal - Hungary) and chromogen for ELISA (Sigma - USA) were analytical grade. Incomplete Freund adjuvant (Sigma - USA) was used for immunisation. For chromatography, Ultrogel AcA 22 (LKB - Sweden) and DEAE-Sephacel (Pharmacia - Sweden) pre-swollen gels were used. The chromatographic system consisted of LKB units: 2137-016 and -026 columns, a MicroPerpex peristaltic pump, Uvicord-S photometer and Ultrograd gardient former. The results of ELISA were measured by manual Microplate photometer (CLS 962) at 492 nm.
Experimental animals
Mature Japanese quails and domestic hens were kept in batteries and fed with commercial laying fodder. One group of geese was kept in natural feeding conditions. The eggs of these birds were collected for the isolation of IgY.
The hens were immunised (0, 2nd and 4th week) with a peptide fragment of calpain, while the geese were immunised with attenuated parvo virus strain (Derzsy-disease virus).
Blood was gathered into heparinized tubes and separated for plasma by centrifugation. Eggs were collected and the egg-yolk samples were diluted by PBS (1:1). All samples were deep-frozen until analysis.
The experiments were carried out with consent of the Animal Experiments Board of the University.
Extraction of IgY from egg yolk
The extraction and purification of the serum-derived immunoglobulin fraction (IgY) in the egg yolks of domestic hens and Japanese quail hens is described herein. The method of extraction consists of four steps:
1. Water dilution of egg yolks (10 ml yolk to 100 ml);
2. Precipitation of lipids by dextran sulphate (10%)(Mw < 500 000) (DS), containing 1 M CaCl2 then centrifugation;
3. Precipitation of proteins by sodium sulphate; and
4. Dialysis of precipitate against PBS.
The purification of dialyzed samples was taken by chromatography, using anion-exchange (DEAE-Seplacel) and gel filtration (Ultrogel AcA 22) techniques.
Results
Our results are presented in the following graphs (Figure 1-4) and in the Table. The Figure 2
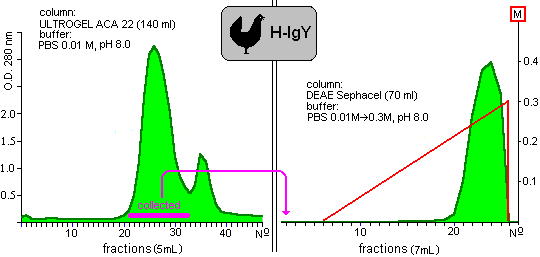 Fig. 2: Purification of yolk immunoglobulin of hens.
Fig. 2: Purification of yolk immunoglobulin of hens.
and Figure 3
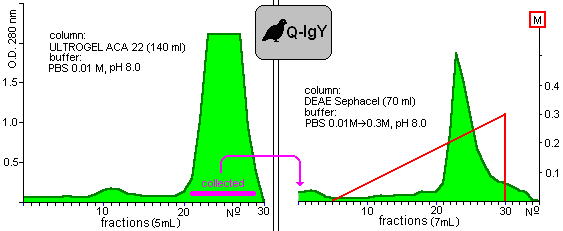 Fig. 3: Purification of yolk immunoglobulin of Japanese quails.
Fig. 3: Purification of yolk immunoglobulin of Japanese quails.
show the chromatograms of proteins which were extracted from hen and quail yolks, respectively. The shapes of chromatograms of extracted proteins were similar in both species either in the gelfiltration or the ion-exchange columns. These indicate the approximately similar properties of isolated proteins. Taking into consideration the initial protein content of yolks, 35% and 27% of the yield of IgY was given in the case of chicken and quail eggs.
ELISA titration curves of hen serum and yolk samples are presented in Figure 4
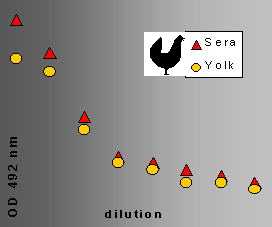 Fig. 4: ELISA titration of serum and yolk anti-Calpain.
Fig. 4: ELISA titration of serum and yolk anti-Calpain.
Hens were immunized with a BSA-conjugate decapeptide (a synthetic Ca-dependent part of Calpain). The serum and the yolk contained the anti-Calpain in nearly equal titre. This indicates a very intensive and efficient transport mechanism from the blood into the follicles (yolk).
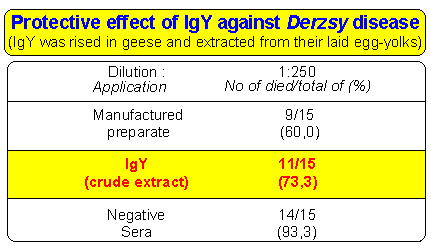 Table: Example of therapeutic use of IgY.
Table: Example of therapeutic use of IgY.
Table shows the protective effect of IgY against Derzsy-disease. Specific IgY rised with immunisation of laying geese. Than it was extracted from their laid egg-yolks. The crude extract was used for cure the artifically infected geese. This preliminary result shows that the crude extract containing IgY against the pathogen agent of Derzsy-disease had a nearly equal protective effect compared to that of manufactured vaccine.
Discussion and Conclusion
The avian egg (mainly poultry) represents a potential new chicken as well as a food of high biological value. The biological value has a new aspect and special meaning nowadays because of the immunoglobulin content of egg yolk. There are a numerous methods and modifications for the isolation of immunoglobulin (IgY) containing protein fraction of yolk.
With the use of thiophilic interaction chromatography for the purification of IgY from egg yolk, the procedure permits the purification to homogeneity of IgY in a single chromatographic step after ammonium sulfate fractionation. They determined that the amount of specific antibody present in egg yolk from an immunized chicken is around 1% of total IgY (6).
Four separation and purification methods in terms of yield, purity, ease of use, potential scaling up and immuno-activity of IgY were compared by Akita and Nakai (1993). The water dilution method (WD) was compared with three other methods, namely, polyethylene glycol (PEG), dextran sulphate (DS) and xanthan gum (Xan) techniques. The WD method gave the highest yield, followed by DS, Xan and PEG methods, in that order. 9.8 mg IgY/ml egg yolk was routinely obtained using the WD method, compared to 4.9 mg IgY/ml egg yolk with the popular PEG method with a purity of 94% and 89%, respectively. All these methods had no adverse effect on the immuno-activities of IgY. WD was also found superior in terms of ease of use and large scale production of IgY. WD method therefore provides a simple, rapid and efficient means of purifying IgY with high activity (1).
The comparative study of some parameters (protein concentration, ovalbumin content, presence of non-specific inhibitors, PAA-gel electrophoresis) showed that the PEG is more efficient and more convenient than the method using organic solvents for the extraction of IgY from normal hen eggs (3).
It was found that the chloroform - polyethylene glycol method yielded 2.5 times more IgY than the polyethylene glycol method. The chloroform had no adverse effect on antibody activity (10).
Eight extraction methods of IgY and method combinations were investigated by PAGE and densitometric analysis. It has been demonstrated that the IgY preparation with DS is very effective, quick and simple to perform. It is well-suited for use in combination with other methods, e.g. ammonium sulfate precipitation (4).
Taking into consideration the previously-mentioned results, a combined (WD+DS) method was used in our experiments. The final yield of IgY were 35% and 27% in the case of chicken and quail eggs. These relatively poor yields are not judged as serious because of the high quantity of AB source (i.e. egg yolk) (13).
There are many examples of the preparation and/or further use of specific antibodies isolated from egg yolk. These ABs have been raised against different pathogen agents e.g. several bacteria: Pseudomonas, Staphylococcus, Salmonella (12), enterotoxigenic E. coli (18), Salmonella typhi-murium (16), and bacterial polysaccharide (alginate) (5), viruses: canine distemper virus (11), Hepatitis-B surface antigen, and potato viruses (5), venoms: rattlesnake - and scorpion venom (14); proteins: transferrin (9), human plasmatic and platelet von Willebrand factor (15), small bio-active peptides: beta-Casokinin-10 (8), and haptens:1,25-dihydroxy-vitamin-D (2), as well.
We have achieved promising results in the case of anti-peptide IgY production (Figure 4)
 Fig. 4: ELISA titration of serum and yolk anti-Calpain.
Fig. 4: ELISA titration of serum and yolk anti-Calpain.
and the therapeutic use of IgY isolated from yolk (Table).
 Table: Example of therapeutic use of IgY.
Table: Example of therapeutic use of IgY.
Immuno-therapy is one of the greatest opportunities for the use of ABs raised in laying birds and isolated from their eggs. Results of Yang et al. (1997) indicated that the antibody IgY can specifically recognize gastrointestinal system cancers. Biological methods for cancer therapies are very important. A small and efficient target carrier is the key component for anti-cancer drugs. For this reason, it may become an important carrier for anti-tumorigenic drugs (17).
With regard to animal welfare (i.e. bleeding), ABs purified from the egg yolks of immunized chickens are an attractive alternative to AB raised from the serum of mammals. Avian egg yolk ABs can play an increasing role as an alternative to classic (mammalian) ABs. They can be used in the fields of immunodiagnostics and therapy as well.
References
- Akita EM, Nakai S. (1993) Comparison of four purification methods for the production of immunoglobulins from eggs laid by hens immunized with an enterotoxigenic E. coli strain.- J Immunol Methods.,160(2):207-214.
- Bauwens RM, Kint JA, Devos MP, Van Brussel KA, De Leenheer AP. (1987) Production, purification and characterization of antibodies to 1,25-dihydroxyvitamin D raised in chicken egg yolk. Clin Chim Acta,170(1):37-44.
- Cuceanu N, Constantinescu C, Ionita E. (1991) Isolation and characterization of egg yolk antibodies IgY from hens immunized with different influenza virus strains.- Roum Arch Microbiol Immunol, 50(3):215-222.
- Fischer M, Hlinak A, Montag T, Claros M, Schade R, Ebner D. (1996) Comparison of standard methods for the preparation of egg yolk antibodies. Tierarztl Prax., 24(4):411-418.
- Gross M, Speck J. (1996) Avian yolk antibodies in diagnosis and research. Dtsch. Tierarztl Wochenschr.,103(10):417-422.
- Hansen P, Scoble JA, Hanson B, Hoogenraad NJ. (1998) Isolation and purification of immunoglobulins from chicken eggs using thiophilic interaction chromatography. J Immunol Methods, 215(1-2):1-7.
- Losonczy S, Batke J. (1997) - Application of specific immuno-globulin (IgY) of egg yolk of birds in the veterinary immuno-diagnosis and immuno-therapy (Review) (in Hungarian) Magy.Áo.Lapja. 119:339-343.
- Meisel H. (1994) Antibodies from egg yolk of immunized hens against a bio-active caseinopeptide (beta-casokinin-10). Biol Chem Hoppe Seyler, 375(6):401-405.
- Ntakarutimana V, Demedts P, van Sande M, Scharpe S. (1992) A simple and economical strategy for downstream processing of specific antibodies to human transferrin from egg yolk. J Immunol Methods,153(1-2):133-140.
- Polson A. (1990) Isolation of IgY from the yolks of eggs by a chloroform polyethylene glycol procedure. Immunol Invest.,19(3):253-258.
- Schmidt P, Hafner A, Reubel GH, Wanke R, Franke V, Losch U, Dahme E. (1989) Production of antibodies to canine distemper virus in chicken eggs for immunohistochemistry. Zentralbl. Veterinarmed. [B], 36(9):661-668.
- Sugita-Konishi Y, Shibata K, Yun SS, Hara-Kudo Y, Yamaguchi K, Kumagai S.(1996) Immune functions of immunoglobulin Y isolated from egg yolk of hens immunized with various infectious bacteria. Biosci.Biotechnol.Biochem.,60(5):886-888.
- Szabó Cs, Bárdos L, Losonczy S, Karchesz K. (1999) Ellenanyag kinyerés tyúk és fürjtojásból. (in Hungarian), Klin.Kísér.Lab.Med., 25:149.
- Thalley BS, Carroll SB.(1990) Rattlesnake and scorpion antivenin from the egg yolks of immunized hens. Biotechnology (N Y),8(10):934-938.
- Toti F, Gachet C, Ohlmann P, Stierle A, Grunebaum L, Wiesel ML, Cazenave JP.(1992) Electrophoretic studies on molecular defects of the von Willebrand factor and platelet glycoprotein IIb-IIIa with antibodies produced in egg yolk from laying hens. Haemostasis, 22(1):32-40.
- Wallmann J, Staak C, Luge E.(1990) A simple method for the isolation of immunoglobulin (Y) from the eggs of immunized hens. Zentralbl Veterinarmed [B],37(4):317-320.
- Yang J, Jin Z, Yu Q, Yang T, Wang H, Liu L.(1997) The selective recognition of antibody IgY for digestive system cancers. Chin.J.Biotechnol.,13(2):85-90.
- Yokoyama H, Peralta RC, Diaz R, Sendo S, Ikemori Y, Kodama Y .(1992) Passive protective effect of chicken egg yolk immunoglobulins against experimental enterotoxigenic Escherichia coli infection in neonatal piglets. Infect.Immun.;60(3):998-1007.
| Discussion Board | Previous Page | Your Poster Session |