Invited Symposium: Molecular Mechanisms of Ageing
| INABIS '98 Home Page | Your Session | Symposia & Poster Sessions | Plenary Sessions | Exhibitors' Foyer | Personal Itinerary | New Search |
Introduction
Senescent growth arrest is a common phenomena observed in a variety of human and animal somatic cells when they are put into culture, and thus the majority of these cells have a finite proliferative potentials (1).Currently, the causative mechanisms for growth arresting signal transduction in sensecent cell is unclear, butĘcandidates for the actual growth arresting factor has been discoverd. We and others isolated and determined the senescent cell inhibitors of cell growth (2,3). The effector molecules are the G1 Cyclin/Cdk inhibiting small proteins that overexpress in senescent cells. We here show the approaches to isolate senescent inhibitors that could be induced or activated during serial proliferation of normal cells. This strategy may also be applied for the isolation of the upstream regulators of the inhibitor expression in senescent cells. The identification of such regulatory factor would clarify the possible molecular link between the inhibitor expression and division counting "clock", e.g. telomerase shortening or decrease of genomic DNA methylations or any other unknown irreversible changes gradually progress in cell aging process.
Materials and Methods
Isolation of sensecent inhibitors by expression screening.
Normal human skin fibroblasts (HCA2) were cultivated until the cells become senescent [PDL(polulation doublings); 87-88].Labeling index as dertermined by 3H-thymidine incorporation indicated <2% of the cells were labeled.The polyA+ RNA was isolated, converted into double strand cDNA, and inserted into mammalian expression vector plasmid pcDSRaD, which resulted in cDNA expression library from sensecent cells. Each E.coli colony carrying cDNA-plasmid was picked randomly and combined into cDNA pools, each composed of five cDNAs. More than 1000 cDNA pools were prepared so far, propagated and individual pool plasmids were isolated.Then the pool plasmids were transfected with DEAE dextran method or microinjected into young, cycling cells along with the transfection marker, pCMVb plasmid. As a control, pcDSRaD and pCMVb are also transfected. During the transient expression of the introduced genes, the cells were labeled with 0.5 micro Ci/ml of 3H-thymidine for 48 hr, fixed, stained with X-gal, and autoradiographed by using Kodak NTB nuclear track emulsion.
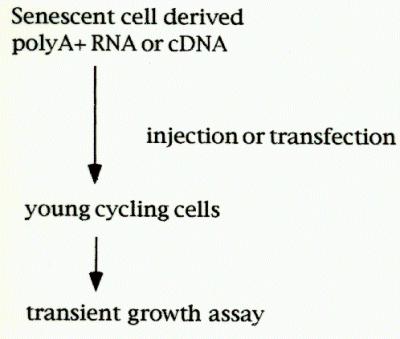 Fig.1:Cloning strategy for senescent inhibitor gene.
Fig.1:Cloning strategy for senescent inhibitor gene.
The percentage inhibiton of DNA synthesis was calculated by the formula [(% labeled nuclei in cells transfected with control plasmid - % labeled nuclei in cells transfected with sensecent cell-derived inhibitors (sdi) plasmid) / % labeled nuclei in cells transfected with control plasmid] X 100. In this way, DNA synthesis activities in b-gal positive cells were determined, and cDNA pools that showed growth inhibitory was further divied into individual plasmid. Finally, candidate cDNA clones (senescent inhibitor of DNA synthesis) were isolated, and their sequences were analyzed.
Northen blot and protein expression analyses.
Northern analysis was performed by standard methods using 1 micro g of polyA+ RNA isolated from young and senescent cells. b-actin or GAPDH expression was also examined as an internal controls for northern analysis. Furthermore, the correlation between p21(sdi1) expression and cell PDs in culture was also analyzed.
Microinjection and antisense expression.
Nuclear microinjection of the isolated plasmids into young cycling or sensecent cells were performed. The plasmid combinations were either pCMVb + pcDSRaD-sdi1(p21) ,or pCMVb + pcDSRaD (control).In each expreriment, 50 micro g/ml of each plasmid was injected. sdi-1(p21) antisense expression construct (pcDSRaD-anti-sdi1) was also microinjected into young and sensecent cells.
Results and Discussion
Cloning strategy for senescent cell inhibitors.
To allow for efficient expression screening of the cDNA library, a very strong promoter was desired. We examined several mammalian expression vectors, and determined that the SRa promoter driving the CAT gene was expressed at high efficieny in young cycling cells. The reporter gene expression was about 20-fold greater than that of the conventional pSV2 vectors.The cDNA plasmids from each pool were co-transfected with a transfection marker plasmid, pCMVb, which allowed us to determine the labeling index of transfected cells.This strategy enabled us to determine three different cDNA pools from senescent cells through five repeated transfections (2).The individual cDNAs from these candidate pools were isolated and further screened, and finally individual inhibitory cDNA sequences were determined and designated as senscent cell-derived inhibitors, sdi-1, -2, and -3.The size of each cDNA insert was 2.1, 1.2 and 2.7 kb, respectively.
 Fig. 2: Sense and anti-sense sdi gene transfections to young cycling cells.
Fig. 2: Sense and anti-sense sdi gene transfections to young cycling cells.
(left) Sense- and antisense- sdi1 expression plasmid (pcDSRaD-sdi1 or pcDSRaD-anti-sdi1) and pCMVb were co-transfected into young cells and % inhibition of DNA synthesis was examined. Lanes 1: pcDSRaD-cat, 2: pcDSRaD-sdi1. 3: pcDSRaD-anti-sdi1, 4: pcDSRaD-sdi2, 5: pcDSRaD-anti-sdi2.(right) Autoradiograph of the sdi1 transfections. A: transfection of control plasmid, B: pcDSRaD-sdi1 transfection. Blue cells (b-gal positive) containing nuclear silver grain indicate DNA synthsis after transfection.
Fig. 2 shows innhibitory effects of sdi-1 and sdi-2 sense and anti-sense transfections to young cycling cells. Overexpression of sdi sequence resulted strong inhibition of young cell growth, whereas that of anti-sense expression showed no inhibition or enhancement. No inhibition was observed in pcDSRaD-cat transfection, indicating that inhibition by sdi was not caused by any full-length cDNA expressed at a high level: not from the overexpression artifacts in the cells. The same results were obtained by microinjection experiments.
Sdi1 expression during cellular senescence.
Changes in sdi1 mRNA expression during cellular senescence was examined by northern analysis. Using full-length cDNA as a probe, we observed the 2.1 kb sdi1 message increased about 20-fold during the in vitro life span of the cells (Fig. 3). While the increase was gradual, the major change in mRNA level occurred during the final few population doublings, correlating closely with expression of senescent phenotype and loss of proliferative potential.
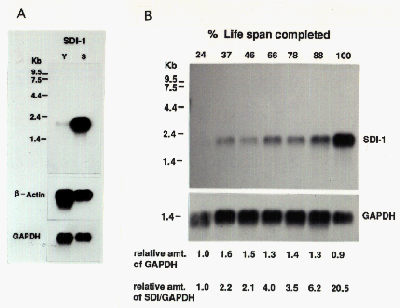 Fig. 3: Sdi1 expression during cellular senescence.
Fig. 3: Sdi1 expression during cellular senescence.
(A) Northern anaysis of sdi1 expression in young and senescent cells. (B) Relative amount of sdi1 expression during in vitro life span.
Role of sdi1 in senescent growth arrest.
Fig. 4 summarizes current understanding of the role of sdi1 in senescent growth arrest. Sdi1 protein appeared to be identical to p21 Cyclin/Cdk inhibitor (4). It could act to inhibit G1 Cyclin/Cdk complexes, therby preventing phosphorylation of the retinoblastoma tumor supressor protein (RB1), which is known tobe hypophosphorylated in senescent cells. This in turn would result in sequestration of E2F transcription factors by hypophosphorylated RB1 and therefore lack of activation of many genes that act during Go/G1 to S transition (5). In fact, knock out experiments of p21 gene revealed that the cells lacking p21 bypassed senescence and continued to replicate (6). However, the cells underwent crisis after 20-30 PD extension, indicating that inactivation of p21 was not a sole requisite for cell immortalization.
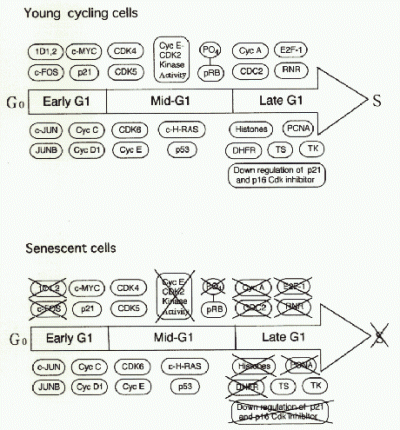 Fig. 4.: Changes of cell cycle regulatory gene expression in growth stimulated senescent cells as compared to growth stimulated young prolifelation competent cells.
Fig. 4.: Changes of cell cycle regulatory gene expression in growth stimulated senescent cells as compared to growth stimulated young prolifelation competent cells.
Signals that cause senescent growth arrest and senescent phenotype.
Changes in many gene expression during cellular aging process has been reported. Theses include extra matrix components (5), cell cycle reguratory genes (7), and transcription factors. p16(ink4), another Cdk inhibitor, is also upregulated in senescent cells (3,8). p21 and p16 could be a direct growth inhibitor whereas others might be a regulator ,or they might be expressed as a result of senescent phenotype.
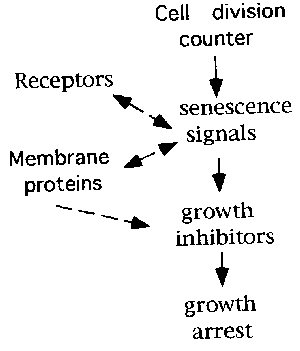 Fig. 5.: Senescence signals in cell aging.
Fig. 5.: Senescence signals in cell aging.
Identification of signal transducing proteins that induce or activate p21 or p16 might reveal possible link between actual growth arrest and gradual irreversible genome changes, shortening of telomere or decrease in DNA methylation, during cell aging.
References
- Norwood, T.H., Smith, J.R., and Stein, G.H.in Handbook of the Biology of aging, Schenider, E.L. and Rowe, J.W., Eds. (Academic press, San Diego, CA, ed. 3, 1990), p.131.
- Noda, A., Ning, Y., Venable, S.F. et al.(1994) Exp. Cell. Res. 211, 90-98.
- Hara, E., Smith, R., Parry, D. et al.(1996) Mol Cell Biol.16, 859-867.
- Hunter, T. (1993) Cell 75, 839-841.
- Smith, J.R. and Pereira-Smith, O.M. (1996) Science 273, 63-67.
- Brown, J.P., Wei, W., and Sedivy, J.M. (1997) Science 277, 831-833.
- Stein, G.H., Drullinger., Robetorye, R.S. et al. (1991) Proc. Natl. Acad. Sci. USA 88, 11012-11016.
- Zindy, F., Quelle, D.E., Roussel, M.F. et al (1997) Oncogene 15, 203-211.
| Discussion Board | Previous Page | Your Symposium |