Invited Symposium: Molecular Mechanisms of Ageing
| INABIS '98 Home Page | Your Session | Symposia & Poster Sessions | Plenary Sessions | Exhibitors' Foyer | Personal Itinerary | New Search |
INTRODUCTION
It would be fair to say that the fundamental cellular processes involved in causing or modulating senescence have not been established for any organism. Indeed, even the existence of mechanisms underlying multiple aging processes or aging processes in different species is a matter of some debate. The existence of single-gene mutations that significantly increase life span in the intensively studied model organism Caenorhabditis elegans (Age mutants, see below) provides an opportunity to identify these critical processes (Lithgow, 1996; Martin et al., 1996; Kenyon, 1996; Johnson, 1997; Wood 1998). A handful of single-gene, loss-of-function mutations that dramatically (>40%) increase the mean life span of the nematode C. elegans (Age mutants) has been identified.
These mutations also convey enhanced resistance to a variety of stresses in non-aged animals, revealing an intimate relationship between increased longevity and increased resistance to environmental stress. The research described here provides insight, at the molecular level, into the mechanisms by which these mutations lead to an increase in stress resistance, and the components of this stress response responsible for increased longevity. The identification of tkr-1 (Murakami and Johnson, 1998), a putative tyrosine kinase whose over-expression leads to stress resistance and increased longevity, provides an important starting point for these studies.
A priori, there is no compelling reason to assume that processes identified in nematodes will necessarily be relevant in other organisms, including humans. However, the fact that all of the Age mutants examined in our lab show an increased ability, even as young animals, to respond to exogenous environmental stress leads us to propose that increased resistance to stress is causally involved in the increased longevity of these mutant strains (Lithgow et al., 1994; 1995; Murakami and Johnson, 1996; Johnson et al., 1996; Lithgow and Kirkwood, 1996, and see Figure 1). Indeed, increased resistance to stress has been proposed to be a mechanism for increased longevity that crosses species barriers (Martin et al., 1996; Johnson et al., 1996; Lithgow and Kirkwood, 1996; Jazwinski, 1996; Guarente, 1997). Longevity mutants in both yeast (Jazwinski 1996; Guarente, 1997) and in Drosophila (Lin et al.,1998) identify gerontogenes that also seem to coordinately regulate stress resistance. Moreover, rodents undergoing dietary restriction (the only process shown to lengthen the life expectancy of mammals) show a significantly increased ability to respond to a variety of environmental stresses (Feuers et al., 1993; Masoro and Austad, 1996; Johnson et al., 1996) and especially reactive oxidants (Sohal and Weindruch, 1996). By understanding the molecular basis of increased stress resistance in C. elegans, we hope to identify components involved in modulating senescence in many organisms, including humans.
GENES EXTENDING LONGEVITY
Gerontogenes are genes that affect the rate of aging (Rattan, 1985). Gerontogenes can be defined operationally to refer to genes that, when altered, lead to a longer than normal maximum life span (Johnson and Lithgow, 1992). We refer to this extended-longevity phenotype as Age. Age mutants. There are four classes of genes in C. elegans in which mutations result in longer adult life expectancy and maximum life span. In three of these classes (the Age, Clk and Spe), the Age phenotype results from hypomorphic or nullomorphic mutations. The fourth class of genes are those which lead to life extension when gene-expression is up-regulated; this class is represented only by tkr-1 (Murakami and Johnson, 1998) at the moment, although other genes are under study (Henderson, Kliminskaya and Johnson, unpublished).
Age. The first gerontogene mutant identified was age-1; it is the only mutant yet studied that was selected based only on its longevity phenotype (Klass, 1983; Duhon et al., 1996). (In the last few weeks Lin et al. (1998) have reported a gerontogene in Drosophila, identified on the basis of extended longevity.) The age-1(hx546) reference allele has a life expectancy 70% longer than wild type and a maximum life span 105% longer (Friedman and Johnson, 1988; Johnson, 1990). age-1 mutations have little effect on fertility, length of reproduction or rate of development (Friedman and Johnson, 1988`; Johnson and Lithgow, 1992; Johnson et al., 1993; Duhon et al., 1996).
age-1(hx546) is resistant to H2O2 (Larsen, 1993), paraquat (Vanfleteren, 1993), and UV (Murakami and Johnson, 1996), is thermotolerant (Lithgow et al., 1994, 1995), and has reduced frequencies of deletions in mitochondrial DNA (Melov et al., 1995). Three other alleles of age-1 were independently isolated on the basis of longevity alone (Duhon et al., 1996) and are also stress resistant (Str) but show subtle variations among themselves. Two more alleles of age-1 have been isolated by selecting for increased thermotolerance (G. Lithgow, personal communication). age-1 mutants are dauer constitutive (Daf-c) only at the semi-lethal temperature of 27C (Malone et al., 1996; Morris et al., 1996). The dauer is an alternative developmental path taken by C. elegans under conditions of crowding or starvation and Daf mutations affect the dauer-formation pathway (Riddle, 1988; Riddle et al., 1997). Mutations in another gene, previously called daf-23, also lead to Age, Daf-c and Str phenotypes (Larsen et al., 1995; Duhon, 1996). daf-23 mutants are Daf-c at 25C and age-1 mutations fail to complement daf-23 mutations (Duhon, 1996; Malone et al., 1996; Morris et al., 1996). Morris et al. (1996) have cloned the daf-23 locus and demonstrated that it shows structural homology with mammalian phosphatidylinositol-3-OH kinase (PI3K) and have also suggested that daf-23 and age-1 are the same gene based on this failure to complement.
Morris et al. (1996) failed to demonstrate any mutation in PI3K for the age-1(hx546) reference allele and no PI3K mutations have been identified in any other allele, as well, despite complete sequencing of at least three of these mutants (Murakami, Kliminskaya and Johnson, unpublished). Morris et al. (1996) also failed to show differential regulation of the putative age-1 gene product either at the mRNA or protein level in mutants previously defined as age-1 which showed the dauer-constitutive phenotype only at 27C. On the basis of these results, the formal possibility exists that the age-1 gene is not the same gene as daf-23 and is not PI3K. (Non-allelic failure to complement has been observed both in the C. elegans spe-6 gene [Varkey et al., 1993] and in other species; for example [Rancourt et al., 1995] suggest that in the mouse HoxB cluster this "non-allelic non-complementation suggests that these two genes function together..."). However, we will assume for the purposes of this paper that PI3K is the protein coded for by the age-1 gene. Mutants in daf-2 are Daf-c at 25 C and result in a more than two-fold extension of life expectancy in the adult phase (Kenyon et al., 1993) and daf-2 interacts with daf-12 to cause an almost four-fold increase in life expectancy (Larsen et al., 1995). daf-2 bears structural homology to the human insulin receptor (Kimura et al., 1997). No other Daf-c genes result in extended longevity (Duhon, 1996); however, mutations in daf-16, a gene coding for a Forkhead class of transcription factor (Ogg et al., 1998; Lin et al., 1998) is necessary for dauer formation and for life extension of age-1(daf-23) and daf-2 (Kenyon et al., 1993; Larsen et al., 1995.?).
Spe. The Spe class of gerontogenes were the second class identified. Two of the six mutant alleles of spe-26, a gene specifying proper segregation of cellular components affecting sperm activation, result in life extensions of about 80% for the hermaphrodite and the mated male (VanVoorhies, 1992; Murakami and Johnson, 1996) although the details are contentious (Gems and Riddle, 1996). No other Spe mutants, except for spe-10 (Duhon, 1996; Cypser and Johnson, in preparation) result in life-extension, although mutants in many do shorten longevity.
Clk. Wong et al. (1995) reported that two of four alleles of clk-1, both of which have altered cell cycle and developmental timing, also have increased life expectancy. The clk-1 gene encodes a 187 amino acid protein (Ewbank et al., 1997) that is probably orthologous to CoQ7, which regulates yeast metabolism (Jonassen, et al., 1998). Ewbank et al (1997) suggest that clk-1 mutants have reduced metabolic rates that are responsible for the extended longevity. Lakowski and Hekimi (1996) have extended these studies to include clk-2, clk-3, and gro-1, all of which have modest (typically 20-30%) extensions of life span, but only for some alleles. There are several additional gerontogene mutants that complement all known Age genes (Duhon et al., 1996; Duhon, 1996; G. Lithgow, personal communication; Y. Yang and D. Wilson, personal communication; G. Ruvkun, personal communication) suggesting that there may be several additional unidentified gerontogenes in C. elegans.
Epistatic interactions among Age mutants. The Age phenotype of age-1 (Kenyon et al., 1993), daf-2 (Larsen et al., 1995; Dorman et al., 1995), spe-26, and clk-1 (Murakami and Johnson, 1996) is suppressed by daf-16 mutations. These results show that the Age phenotype of these mutants is dependent on a functional DAF-16 protein and suggest a possible involvement of the dauer-formation pathway in life extension (Kenyon et al., 1993; Dorman et al., 1995; Duhon, 1996). Lakowski and Hekimi (1996) reported that double mutants of daf-2 and clk-1 extend life expectancy almost five-fold and suggested that clk-1 identifies a pathway distinct from that identified by daf-2 and age-1.
RESISTANCE TO ENVIRONMENTAL STRESS
All of the gerontogene mutants in C. elegans that have been tested are more resistant to environmental stresses such as reactive oxygen species (ROS) (Larsen, 1993; Vanfleteren, 1993), high temperature (Lithgow et al., 1994, 1995) and UV radiation (Murakami and Johnson, 1996). Increased thermotolerance is seen in all of the life-extension mutants tested; however, daf-4 and daf-7 mutants (Daf-c) are thermotolerant but are not Age (Lithgow et al., 1995). Of the three stressors, UV is the most consistent predictor of life extension (Murakami and Johnson, 1996) even showing correspondence between different alleles of spe-26 and clk-1. The increased UV resistance of age-1/(daf-23), daf-2, spe-26 and clk-1 is suppressed by daf-16 (Murakami and Johnson, 1996); all Str traits tested are also suppressed by daf-16. These results are summarized in Figure 1.
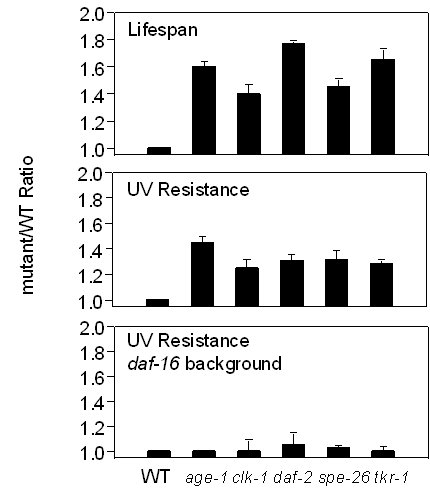 Fig. 1: Summary of mean life spans and UV resistance of Age mutants.
Fig. 1: Summary of mean life spans and UV resistance of Age mutants.
Figure 1. Observations are presented as Age mutant/wild type ratios, as data are compiled from independent studies. Typical data are shown. The life expectancy data of each strain are from Johnson, 1990 (age-1), Wong et al., 1995 (clk-1), Larsen et al., 1995 (daf-2), Murakami and Johnson, 1996 (spe-26), and Murakami and Johnson, 1998 (tkr-1). All the UV-resistance data are from Murakami and Johnson, 1996. The alleles shown here are age-1 (hx546) , clk-1(e2519) , daf-2(e1370) , spe-26(hc138) , and tkr-1(zIs3000). The increased resistance to a variety of environmental stressors and the putative roles of age-1/daf-23, daf-2, and daf-16 in a signal-transduction pathway involving the phosphoinositides are consistent with a model in which life span is determined as a result of altered sensitivity to the environment and increased ability to resist or repair environmental damage (Johnson et al., 1996). The facts that the spe-26 and clk-1 Age alleles (but not the non-Age alleles) are also resistant to UV and UV resistance of spe-26 are suppressed by daf-16 without altering fertility (Murakami and Johnson, 1996), are consistent with increased resistance playing a causal role in the life-extension of these mutants.
THE NOVEL GERONTOGENE TKR-1
As delineated above, there is extensive evidence supporting stress resistance as a central mechanism generating the long-life phenotype of Age mutations. However, competing (if not necessarily mutually exclusive) hypotheses have been proposed. These include the concept of a central “life span” clock that can be genetically reset by mutation (Kenyon, 1996), as well as the view that Age mutations convey longevity primarily by altering cellular metabolism (Guarente, 1997; Ogg et al., 1997). clk-1 animals are longer lived, have altered cell cycles and other rhythmic behaviors (Ewbank et al., 1997), and the clk-1 gene is probably orthologous to CoQ7, which regulates yeast metabolism (). The sequence similarities of daf-2 to human insulin receptor, and age-1 to human PI3 kinase, have led to the interpretation that these genes regulate glucose metabolism (Kimura et al., 1997).
However, no direct metabolic or biochemical measurements have been made to critically test these assumptions. If these Age mutations do in fact regulate metabolism, the stress resistance resulting from these mutations may be induced by mechanisms similar to those that induce stress resistance in dietary-restricted rodents.
As described in Preliminary Results, we have identified a novel gerontogene, tkr-1, that increases stress resistance and longevity when over-expressed in transgenic animals. Importantly, these transgenic animals do not show altered developmental rates, and show normal induction of dauer larvae. Nevertheless, the stress resistance and longevity of these animals is suppressed by mutations in daf-16 (as is observed for age-1, daf-2 and clk-1), therefore formally placing tkr-1 in a common pathway with other gerontogenes. These results strongly support stress resistance as the general mechanism of life extension in C. elegans. These results also illustrate the importance of both establishing the molecular mechanisms by which tkr-1 over-expression induces stress resistance and life extension, and identifying the genes involved in regulating and enacting stress response in C. elegans.
The Cellular Stress Response in Eukaryotes.
Response to stress has not been well studied in C. elegans. However, the fundamental importance of stress responses for all organisms makes it highly likely that insights gained from other organisms can be applied to C. elegans, and vice versa. Shown in Figure 2 is a generalized scheme illustrating signal transduction components implicated in the cellular stress response of yeast and mammalian cells (reviewed in Lewis et al., 1997), as well as specific components identified for mammalian UV response. A central event in the cellular stress response is the activation of a kinase of the MAPK class and the subsequent phosphorylation and activation of appropriate transcription factors. For example, in mammalian cells UV exposure leads to the activation of JNK (SAPK) kinase, and the subsequent phosphorylation and activation of cJUN. Although the activation of kinase cascades has been established for a number of stresses (e.g., UV, osmotic, and oxidative), the initial sensors for these stresses have not been established, nor have mechanisms for down-regulation of stress responses been commonly examined.
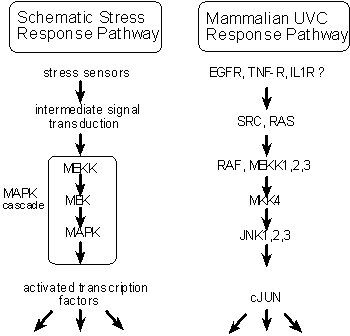 Fig. 2: Signaling cascade for stress.
Fig. 2: Signaling cascade for stress.
Figure 2. (Left) Schematic for signaling cascade leading to induction of genes modulating response to stress. (Right) Characterized signal transduction cascade induced by UV exposure in mammalian cells.
Of particular interest to this hypothesis are studies implicating Epidermal Growth Factor Receptor (EGFR), a classic receptor tyrosine kinase, in the response to UV stress in mammalian cells. Exposure of HeLa cells to short-wavelength ultraviolet light induces immediate, suramin-inhibitable, tyrosine phosphorylation of EGFR, and expression of a dominant negative EGFR inhibits UV-induced gene expression (Sachsenmaier et al., 1994). Exposure to UV light has also been shown to induce the aggregation of EGFR, as well as IL-1 and TNF receptors (Rosette and Karin, 1996); receptor aggregation is known to be sufficient to induce activation of these receptors in the absence of ligand (Heldin, 1995). These results indicate that receptor tyrosine kinases can participate in stress responses in a ligand-independent manner. We postulate that tkr-1 functions in a similar manner in C. elegans.
Why Do Gerontogenes Exist?
The existence of loss-of-function mutations in age-1 and daf-2 that extend life span has led to the straightforward, if non-intuitive, view that the wild-type function of these genes is to reduce life span (Friedman and Johnson, 1988). The actual function of these genes becomes more easily understood when their proposed role in regulating stress response is considered. In this context, a wild-type function of these genes is to down-regulate the stress response, thereby conserving cellular resources when stressful conditions are not present. Although age-1 mutants are not obviously impaired in fertility under laboratory conditions (Johnson et al., 1993), these mutants are readily out-competed when propagated with wild-type animals under an alternating feeding/starvation cycle regime that more closely mimics natural conditions (Lithgow et al., personal communication). This result implies that loss of age-1 function has a fitness cost, and explains the evolutionary selection for the wild-type function of this gene. These results also suggest that the capacity to prevent or repair macromolecular damage has not been maximized by evolution (Kirkwood, 1977; Lithgow and Kirkwood, 1996).
Additional capabilities are latent within the animal and can be stimulated, leading to increased life span (Johnson et al., 1996). In contrast to the function of age-1 and daf-2, the proposed wild-type function of tkr-1 is to up-regulate stress-responsive genes. (We would predict that tkr-1 over-expression, which increases longevity, may also reduce overall fitness.) It should be remembered that the longevity phenotype assayed in our studies is highly artificial, in the sense that the nematode life spans measured in culture are likely never attained in natural populations, and are completely irrelevant in an evolutionary sense. (We note that human life spans attained in today’s society are similarly evolutionarily irrelevant.) In summary, gerontogenes exist because there has been no selection for maximum post-reproductive life span, and life spans can be significantly increased by changes in genetically-controlled physiological functions.
REFERENCES
Dorman, J. P., Albinder, B., Shroyer, T. and Kenyon, C. (1995) The age-1 and daf-2 genes function in a common pathway to control the lifespan of Caenorhabditis elegans. Genetics, 141:1399-1406.
Duhon, S. A. (1996) The isolation and characterization of Age mutants in the nematode Caenorhabditis elegans. Thesis, University of Colorado at Boulder.
Duhon, S. A., Murakami, S. and Johnson, T. E. (1996) Direct isolation of longevity mutants in the nematode Caenorhabditis elegans. Develop. Genet., 18: 144-153.
Ewbank, J. J., Barnes, T. M., Lakowski, B., Lussier, M., Bussey, H., and Hekimi, S. (1997) Structural and functional conservation of the Caenorhabditis elegans timing gene clk-1. Science, 273:980-983.
Feuers, R. J., Weindruch, R. and Hart, R. W. (1993) Caloric restriction, aging, and antioxidant enzymes. Mut. Res., 295: 191-200.
Friedman, D. B., Johnson, T. E. (1988) A mutation in the age-1 gene in Caenorhabditis elegans lengthens life and reduces hermaphrodite fertility. Genetics, 118: 75-86.
Gems, D. and Riddle, D. R., (1996) Mating but not gamete production reduces longevity in Caenorhabditis elegans. Nature, 379:723-725.
Guarante, L. (1997) What makes us tick? Science, 275:943-944
Heldin, C. H. (1995) Dimerization of cell surface receptors in signal transduction. Cell, 80: 213-223.
Jazwinski, S. M. (1996) Longevity, genes and aging. Science, 273:54-58.
Johnson, T. E. (1990) The increased life span of age-1 mutants in Caenorhabditis elegans results from lowering the Gompertz rate of aging. Science, 249: 908-912.
Johnson, T. E. (1997) Genetic influences on aging. Exp. Gerontol. 32: 11-22.
Johnson, T. E. and Lithgow, G. J. (1992) The search for the genetic basis of aging: The identification of gerontogenes in the nematode Caenorhabditis elegans. J. Amer. Ger. Soc., 40:936-945.
Johnson, T. E., Lithgow, G. J. and Murakami, S. (1996) Hypothesis: Interventions that increase the response to stress offer the potential for effective life prolongation and increased health. J. Gerontol. Bio. Sci,. 51: B392-B395.
Johnson, T. E., Tedesco, P. M. and Lithgow, G.J. (1993) Comparing mutants, selective breeding, and transgenics in the dissection of aging processes of Caenorhabditis elegans. Genetica, 91:65-77.
Jonassen, T. Proft, M., Randex-Gil, F. Schultz, J. R. Marbois, B. N., Entian, K. D. and Clarke, C. F (1998) Yeast Clk-1 homologue (Coq7/Cat5) is a mitochondrial protein in coenzyme Q synthesis. J Biol Chem 273:3351-7.
Kenyon, C. (1996) Environmental factors and gene activities that influence life span. In C. elegans II. (Riddle, D. L., Blumenthal, T., Meyer, B. J. and Priess, J. R., Eds.), Cold Spring Harbor Press, Cold Spring Harbor, NY, pp. 791-813.
Kenyon, C., Chang, J., Gensch, E., Rudner, A., and Tabtiang, R. (1993) A C. elegans mutant that lives twice as long as wild type. Nature, 366:461-464.
Kimura, K. D., Tissenbaum, H.A., Liu, Y., and Ruvkun, G. (1997) daf-2, an insulin receptor-like gene that regulates longevity and diapause I Caenorhabditis elegans. Science, 277: 942-946.
Kirkwood, T. B. L. (1977) Evolution of aging. Nature, 270: 301-304.
Klass, M. R. (1983) A method for the isolation of longevity mutants in the nematode Caenorhabditis elegans and initial results. Mech. Ageing Dev., 22:279-286.
Lakowski, B. and Hekimi, S. (1996) Determination of life-span in Caenorhabditis elegans by four clock genes. Science, 272:1010-1013.
Larsen, P. L. (1993) Aging and resistance to oxidative damage in Caenorhabditis elegans. Proc Natl Acad Sci USA, 90: 8905-8909.
Larsen, P. L., Albert, P. S., Riddle, D. L. (1995) Genes that regulate both development and longevity in Caenorhabditis elegans. Genetics, 139:1567-1583.
Lewis, T.S., Shapiro, P.S., and Ahn, N. (1997) Signal transduction through map kinase cascades. Adv. Cancer Res., 74: 49-139.
Lin, Y.-J., Seroude, L. and Benzer, S. (1998) Extended life-span and stress resistance in the Drosophila mutant methuselah. Science, 282: 943-946.
Lin, K., Dorman, J. B., Rodan, A., and Kenyon, C. (1997) daf-16: An HNF-3/forkhead family member that can function to double the life-span of Caenorhabditis elegans. Science, 278: 1319-1322.
Lithgow, G. J. (1996) The molecular genetics of Caenorhabditis elegans. In Handbook of the Biology of Aging, Fourth Edition, (Schneider, E. L. and Rowe, J. W., Eds.) Academic Press, New York, pp. 55-73.
Lithgow, G. J. and Kirkwood, T. B. L. (1996) Mechanisms and evolution of aging. Science, 273:321-324.
Lithgow, G. J., White, T. M., Hinerfeld, D. A., and Johnson, T. E. (1994) Thermotolerance of a long-lived mutant of Caenorhabditis elegans. J. Gerontol. Bio. Sci., 49:B270-B276.
Lithgow, G. J., White, T. M., Melov, S., Johnson, T. E. (1995) Thermotolerance and extended life-span conferred by single-gene mutations and induced by thermal stress. Proc. Natl. Acad. Sci. USA., 92:7540-7544.
Malone, E. A., T. Inoue and Thomas, J. H. (1996) Genetic analysis of the roles of daf-28 and age-1 in regulating Caenorhabditis elegans dauer formation. Genetics, 143:1193-1205.
Martin, G. M., Austad, S. N. and Johnson, T. E. (1996) Genetic analysis of aging: Role of oxidative damage and environmental stresses. Nat. Genetics, 13:25-34.
Masoro, E. J. and Austad, S. N. (1996) The evolution of the antiaging action of dietary restriction: A hypothesis. J. Gerontol., Biol. Sci. 51:B387-B391.
Melov, S., Lithgow, G. J., Fischer, D. R., Tedesco, P. M. and Johnson, T. E. (1995) Increased frequency of deletions in the mitochondrial genome with age of Caenorhabditis elegans. Nucleic Acids Res., 23:1419-1425.
Morris, J. Z., Tissenbaum, H. A., and Ruvkun, G. (1996) A phosphatidylinositol-3-OH kinase family member regulating longevity and diapause in Caenorhabditis elegans. Nature, 382:536-539.
Murakami, S. and Johnson, T. E. (1996) A genetic pathway conferring life extension and resistance to UV stress in Caenorhabditis elegans. Genetics, 143:1207-1218.
Murakami, S. and Johnson, T. E. (1998) Life extension and stress resistance stress in Caenorhabditis elegans modulated by the tkr-1 gene. Curr. Biol., 8:1091-1094.
Ogg, S., Paradis, S., Gottlieb, S., Patterson G. I., Lee L., Tissenbaum H. A., and Ruvkun G. (1997) The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signal in C. elegans. Nature Genetics, 389: 994-999.
Rancourt, D. E., Tsuzuki, T. Capecchi, M. R., (1995) Genetic interaction between hoxb-5 and hoxb-6 is revealed by nonallelic noncomplementation. Genes Dev 9:108-22
Rattan, S. I. S., (1985) Beyond the present crisis in gerontology. Bioessays, 2:226-228.
Riddle, D. L., (1988) The dauer larva. In The Nematode Caenorhabditis elegans (ed. W. B. Wood), Cold Spring Harbor Press, Cold Spring Harbor, NY., pp. 393-412.
Riddle, D. L. and Albert, P. S. (1997) Genetic and environmental regulation of dauer larva development. In C. elegans II. (Riddle, D. L., Blumenthal, T., Meyer, B. J. and Priess, J. R., Eds.), Cold Spring Harbor Press, Cold Spring Harbor, NY., pp.739-768.
Sachsenmaier, C., Radler-Pohl, A. Zinck, R., Nordheim, A., Herrlich, P. and Rahmsdorf, H. J. (1994) Involvement of growth factor receptors in the mammalian UVC response. Cell, 78: 963-972.
Sohal, R. C., and Weindruch, R. (1996) Oxidative stress, caloric restriction, and aging. Science, 273:59-63.
Vanfleteren, J. R. (1993) Oxidative stress and ageing in Caenorhabditis elegans. Biochem. J., 292: 605-608.
VanVoorhies, W. A. (1992) Production of sperm reduces nematode lifespan. Nature, 360:456-458.
Varkey, J. P., Muhlrad, P. J., Minniti, A. N., Do, B. and Ward, S. (1995) The Caenorhabditis elegans spe26 gene is necessary to form spermatids and encodes a protein similar to the actin-associated proteins kelsh and scruin. Genes Devel., 9:1074-1086.
Wong, A. Boutis, P. and Hekimi, S. (1995) Mutations in the clk-1 gene of Caenorhabditis elegans affect developmental and behavioral timing. Genetics, 139:1247-1259.
Wood, W. B. (1998) Aging of C. elegans: mosaics and mechanisms. Cell, 95:147-150.
| Discussion Board | Previous Page | Your Symposium |