Invited Symposium: Na-H Exchangers and Intracellular pH Regulation
| INABIS '98 Home Page | Your Session | Symposia & Poster Sessions | Plenary Sessions | Exhibitors' Foyer | Personal Itinerary | New Search |
Introduction
Na+/H+ antiporters are integral membrane proteins residing in the plasma membranes of different cells. They fall into two distinct classes depending on their physiological role. One type is Na+/H+ exchange in vertebrates that normally functions to remove excess internal acid from the cytoplasm in exchange for external sodium ions. The sodium gradient created by Na+/K+ ATPase serves as the energy source to drive this process. Another class of Na+/H+ antiporters functions in a similar fashion but normally transports ions in the opposite direction.
Bacterial Na+/H+ antiporters normally expel intracellular Na+ by using the preexisting protonmotive force. The main bacterial Na+/H+ antiporter of Escherichia coli is NhaA. His225 of NhaA determines the pH profile of activity of the protein whereas the other seven His residues are not essential for NhaA. Substitution of His 225 with an acidic residue (Asp) shifts the pH optimum toward a more alkaline pH. Substitution of His 225 with a basic residue (Arg) results in a more acidic pH optimum of activity of the protein. The H225A mutation inactivates the antiporter almost completely.
While His residues are important in the pH activity profile of NhaA, mutation of His residues in the mammalian Na+/H+ antiporter does not affect H+ transport. Thus there appears to be a clear difference in the mechanism by which the two types of Na+/H+ antiporter regulate their pH optimum and in the functional role of His residues in these proteins.
Other amino acids residues which may be critical in Na+/H+ antiport are negatively charged residues located in transmembrane segments. Such residues are known to be good candidates for binding and translocation of cations and could coordinate of Na+ and H+ binding. In this regard, three Asp residues have been shown to be crucial for Na+/H+ antiport by NhaA in E. coli and by the related protein in Vibrio alginolyticus . These negatively charged residues are conserved in their position in these two species and are located in neighboring transmembrane segments of the protein. They form a characteristic intramembrane pattern herein referred to as a '3-D motif'. An examination of protein sequences involved shows that this '3-D motif' or a variation thereof is present in all NhaA-like proteins and in yeast Na+/H+ antiporters despite the absence of a strong homology between other residues of these regions (Fig. 1).
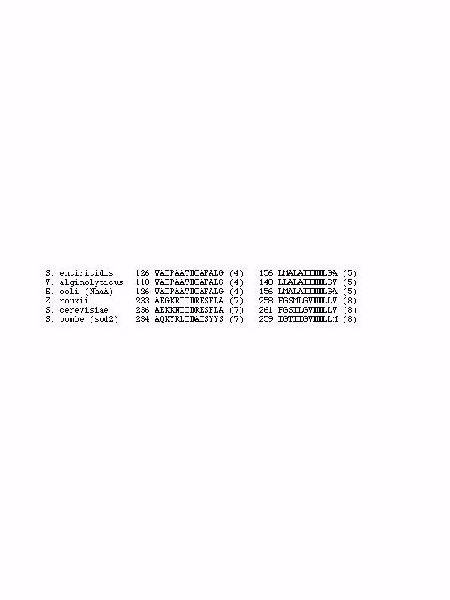 Fig. 1: Alignment of Prokaryotic and Eukaryotic Na+/H+ antiporters. Bold residues indicate conserved amino acids that may be important in cation binding. Numbers preceding sequences indicate the number of the first amino acid in the sequence shown. Numbers in brackets following the sequence indicates the putative transmembrane segment in which the fragment is found. For S. pombe the topology was assigned using the program Topopredict II .
Fig. 1: Alignment of Prokaryotic and Eukaryotic Na+/H+ antiporters. Bold residues indicate conserved amino acids that may be important in cation binding. Numbers preceding sequences indicate the number of the first amino acid in the sequence shown. Numbers in brackets following the sequence indicates the putative transmembrane segment in which the fragment is found. For S. pombe the topology was assigned using the program Topopredict II .
It is noteworthy that His225 which functions as a pH sensor in NhaA, is the first His residue following the intramembrane '3-D motif'. In all the bacterial NhaA-like proteins, there is an equivalent His residue, approximately 60 residues downstream of the last functionally important Asp of the '3-D motif'. Sod2 is the Na+/H+ antiporter present in the fission yeast Schizosaccharomyces pombe and is the sole molecular mechanism that removes Na+ ions from the cytoplasm. It does so at the expense of the proton gradient (DpH) created by the plasma membrane ATPase. Related homologous proteins have been documented in Zygosaccharomyces rouxii and in Saccharomyces cerevisiae.
Although there is no significant overall homology between sod2 and NhaA, sod2 and all the yeast Na+/H+ antiporters contain the putative intramembrane '3-D motif' (Fig. 1). In addition there is a His residue that is located 100 amino acid residues downstream of the Asp pair of the '3-D motif' of sod2. It is therefore possible that the functional analogy between bacterial and fungal Na+/H+ antiporters is based on a common molecular mechanism involving the same amino acids at critical positions, even though overall, the proteins are not highly homologous to each other. The residues critical for transport and for the pH optimum of the proteins may be distributed in similar locations executing analogous functions. To test this hypothesis, we performed mutational analysis of sod2. All His residues of sod2 as well as three conserved Asp residues within hydrophobic amino acid sequences (parts of '3-D motif'), were subjected to site-specific mutagenesis. The data obtained supports the idea that sod2 and NhaA share common molecular mechanism of ion exchange involving the '3D motif' and a His residue following this motif.
| <= Abstract | INTRODUCTION | Materials & Methods => |
| Discussion Board | Next Page | Your Symposium |