Invited Symposium: Angiotensin Receptors
| INABIS '98 Home Page | Your Session | Symposia & Poster Sessions | Plenary Sessions | Exhibitors' Foyer | Personal Itinerary | New Search |
Introduction
The octapeptide hormone angiotensin II (AngII) exerts its numerous physiological effects on cardiovascular, endocrine and neuronal systems by interacting with two pharmacologically distinct types of receptors. The type 1 receptor (AT1)mediates most of the known physiological actions of AngII whereas the type 2 receptor (AT2) appears to act as a physiological antagonist of AT1-receptor effects (1,2). The AT1 and AT2 receptors have been cloned and are members of the G-protein coupled receptors family (GPCR). The elucidation of primary structures of numerous GPCRs permitted to identify domains in receptors directly involved in ligand binding. As can be seen in Figure 1, we have developed a direct approach to identify the contact points between AngII and its receptors. We previously reported the covalent labeling of AngII receptors with radioactive and photosensitive analogs of AngII (3-6). In the present study, after covalent labeling, AngII receptors were subjected to enzymatic or chemical fragmentation. The fragments were then resolved by SDS-PAGE electrophoresis followed by autoradiography. The sequence of the labeled fragment was deduced from its apparent size. In complement to mutagenesis studies, our direct approach for the identification of ligand binding domains of AngII receptors will provide very useful information for drawing three dimensional models of ligands-receptors complexes.
Materials and Methods
Photoaffinity labeling.
Endogenously expressing or transfected cells expressing AT1 or AT2 receptors were incubated in binding medium in the presence of photoreactive analogs of AngII containing the p-benzoylphenylalanine (Bpa) moiety at position 1,3 or 8. After 45 min at room temperature, cells were washed and irradiated for 60 min at 0 °C under filtered UV light (365 nm). Cells were then scraped off the plate and solubilized in a medium containing 100 mM Na2 HPO4, pH 8.5, 25mM EDTA, 1 mg/ml soybean trypsin inhibitor and 1% (v/v) Nonidet P-40. The supernatant was kept at -80 °C until further analysis.
Endoglycosidase digestion.As can be seen in Figure 2 and in Figure 3, AT1 and AT2 receptors are glycoproteins possessing multiple sites of cleavages specific for enzymatic or chemical digestions as indicated. Solubilized receptors were incubated for 2h at 37 °C in the presence of PNGase-F (33 units/ml) in a medium containing 0.2% (v/v) NP-40.
Receptor fragmentation.For enzymatic digestions, photolabeled receptors were resuspended in digestion buffer containing 100mM NH4 HCO3, pH 8.0, 0.1% (w/v) SDS and 800 µg/ml V8 protease for 4 days at room temperature. Photolabeled receptors were also digested for 18-24h at 37 °C with 40 µg/ml Endo Lys-C in digestion buffer composed of 25mM Tris-HCl, pH 8.5, 1mM EDTA and 0.1% (w/v) SDS. All digestions were terminated by adding an equal volume of 2 x Laemmli buffer and boiling the samples for 3 min. When consecutive digestions were needed, products of the first digestion were identified by SDS-PAGE followed by autoradiography and recovered from non-fixed gels by passive elution for 12-18h at 37 °C with 500 µl of digestion buffer. Extracted proteins were lyophilised and submitted to chemical or enzymatic digestion as described.
For CNBr hydrolysis, photolabeled receptors were resuspended in 70% (v/v) trifluoroacetic acid and CNBr was added to a final concentration of 100 mg/ml. Samples were incubated at room temperature, in the dark, for 24-36h. Reactions were terminated by dilution with water. For TNB-CN hydrolysis, photolabeled receptors were resuspended in a buffer containing 100mM Tris-HCl, pH 8.5, 5 mM dithiothreitol, 0.1% (w/v) SDS and a 5-fold excess of TNB-CN (25 mM) over Thiol, and pH was readjusted, if necessary, to 8.5 with Na OH. The mixture was allowed to stand at room temperature for 60 min. An equal volume of 200 mM sodium borate pH 10.5 was added and the reaction lasted for 2h at 70 °C. TNB-CN hydrolysis was terminated by adding an equal volume of 2 x Laemmli buffer.
Analysis of products of proteolysis and chemical cleavage.The products of proteolysis and deglycosylation were analyzed by SDS-PAGE using 16.5% acrylamide Tris-Tricine gels (BioRad) followed by autoradiography on X-ray films (Kodak BIOMAX MS). 14C-labeled low molecular protein standards (Gibco BRL) were used to determine apparent molecular masses. Running conditions, fixation and coloration of gels were done according to the manufacturer's instructions.
Results
As can be seen in Figure 4, AT1 receptor is specifically photolabeled with three AngII analogs bearing the photosensitive moiety (Bpa) at position 1, 3 or 8. The labeling was inhibited in the presence of a high concentration of non radioactive AngII and in the presence of 10 µM losartan, a selective antagonist of AT1 receptor. When the [BPA8]AngII-AT1 receptor complex was digested with CNBr under the conditions described in "Methods", a 7.5 kDa, non-glycosylated (sharp band), fragment was produced as can be seen in Figure 5A. PNGase-F digestion of this fragment had no effect on its electrophoretic mobility (results not shown). Considering the location of the methionine residues at positions 243, 284 and 334 in the AT1 receptor sequence (see Figure 2). This apparent molecular mass identifies the fragment to the Pro285-Met334 polypeptide in the seventh transmembrane domain/cytoplasmic tail of AT1 receptor. After passive elution, Endo-LysC digestion of this 7.5 kDa fragment produced a shorter 3.6 kDa fragment (see Figure 5A). Since Endo Lys-C specifically cleaves on the carboxylic side of lysine residues, the 3.6 kDa fragment corresponds to the Pro285-Lys307 polypeptide. The 7.5 kDa CNBr fragment was also submitted to chemical hydrolysis with TNB-CN which cleaves at the amino-side of cysteine residues. Results shown in Figure 5B indicate that the 7.5 kDa CNBr fragment contains at least one cysteine residue since it was converted to a 3.1 kDa fragment upon cleavage with TNB-CN. Indeed the fragment Pro285-Lys307 contains two cysteine residues at positions 289 and 296 (see Figure 2). Complete cleavage at these residues should produce either the labeled peptide Pro285-I288 or the labeled peptide Cys289-Asn295. Both fragments are expected to migrate with estimated Mrs of 1.7 and 2.2 kDa respectively. However we could only detect a 3.1 kDa fragment probably corresponding to the partial hydrolysis of the peptide at position 296 which would generate the labeled Pro285-Asn295 fragment. The possibility that the labeled 3.1 kDa fragment corresponds to the peptide Cys296-Met334 must be excluded because it would be expected to migrate with an estimated Mr of 6.4 kDa. Taken together, these results indicate that [Bpa8]AngII labels the fragment encompassing residues Pro285-Asn295 within the seventh transmembrane domain of AT1 receptor.
To further narrow down the size of the label-bearing receptor segment and actually to identify which amino acid, within the identified fragment, interacts with the photoreactive analog, we used an approach that takes advantage of the Edman degradation. As can be seen in Figure 6, the strategy consists first, in acetylating the photolabeled receptor with sulfosuccinimidyl acetate. This acetylation step is essential to block the N-terminus of the photoligand peptide in order to render it resistant to Edman reaction. After the acetylation step, the photolabeled receptor was cleaved with CNBr to release the Pro285-Met334-photolabeled fragment (with a free N-terminus), that was submitted to successive cycles of Edman degradation. As can be seen in Figure 7, the radioligand was released on the ninth and the tenth cycles, identifying Phe293 and Asn294 as contact points for the C-terminal part of AngII on AT1 receptor.
Using that general strategy, we identified the contact points of [Bpa1]AngII, [Bpa8]AngII and [Bpa3]AngII on AngII receptors. As can be seen on Figure 8, [Bpa1]AngII and [Bpa3]AngII interacted with a common segment of AT1 receptor encompassing His166-Lys199 whereas [Bpa8]AngII, as previously mentioned, interacted with the segment Pro285-Asn295. As can be seen on Figure 9, [Bpa1]AngII and [Bpa3]AngII again interacted with a common segment located in the N-terminal tail of AT2 receptor encompassing Asp3-Glu30, whereas [Bpa8]AngII interacted with a segment located in the third transmembrane domain of AT2 receptor encompassing Phe129-Met138.
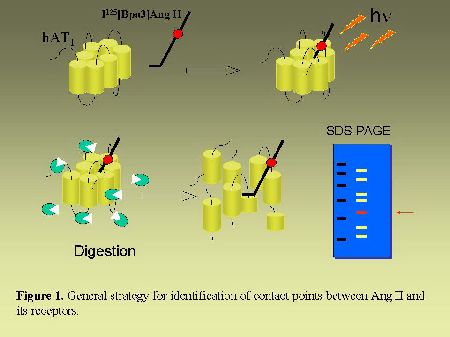 Fig. 1.
Fig. 1.
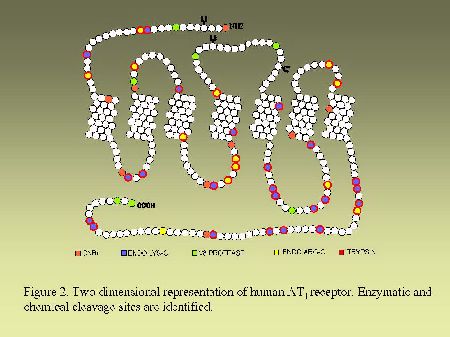 Fig. 2.
Fig. 2.
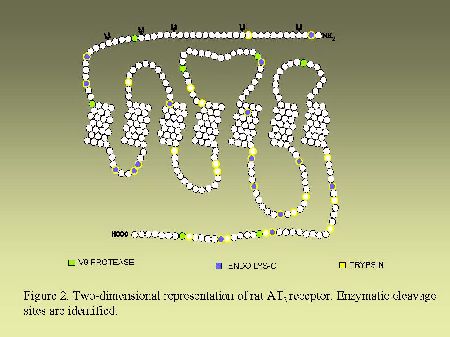 Fig. 3.
Fig. 3.
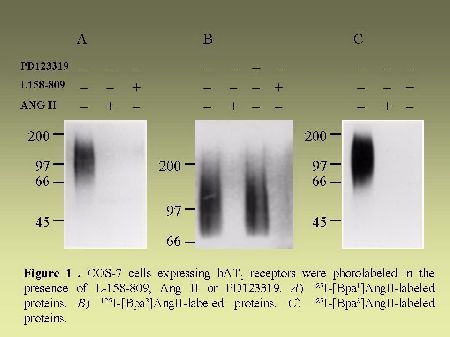 Fig. 4.
Fig. 4.
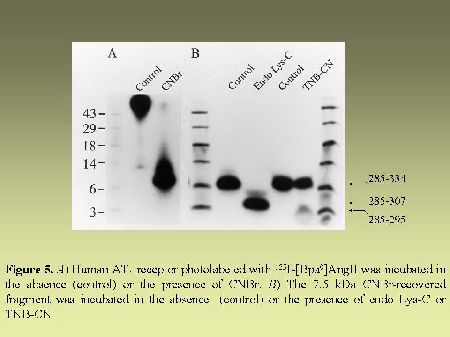 Fig. 5.
Fig. 5.
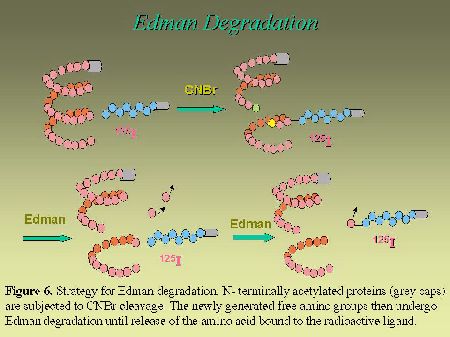 Fig. 6.
Fig. 6.
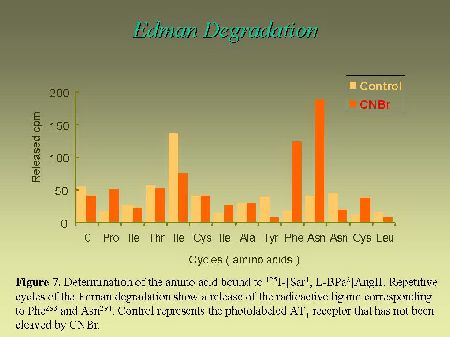 Fig. 7.
Fig. 7.
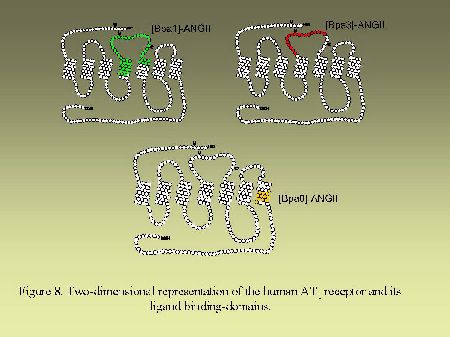 Fig. 8.
Fig. 8.
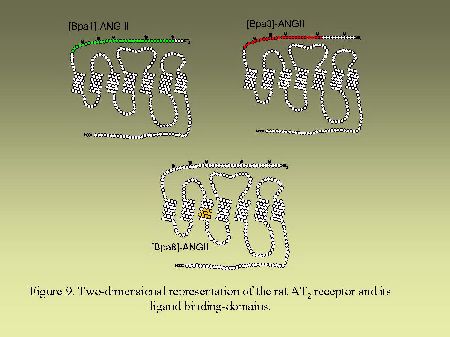 Fig. 9.
Fig. 9.
Discussion and Conclusion
Numerous structure-activity studies performed on AngII have demonstrated that the aromatic ring of Phe8 at the C-terminus of the peptide carries the information needed for the biological response (7). In the present work, we have used a photoaffinity labeling approach to identify contact points between AngII receptors and some of their peptide ligands. Binding domains on the AT1 receptor have also been extensively studied with mutagenesis approaches (8,9). Activation of the AT1 receptor appears to require the interaction of Phe8 side chain of AngII with His256 in the sixth transmembrane domain of the receptor, an interaction which is stabilized by docking the alpha-carboxylic group of Phe8 to Lys199 in the fifth transmembrane domain of the receptor (10,11). It was also suggested that the aromatic Trp253 in the sixth transmembrane domain stabilizes an ionic bridge formed between Lys199 in the fifth transmembrane of AT1 receptor and the carboxylate anion of Phe8 of AngII (12). The C-terminal residue of AngII would therefore be confined within a binding pocket circumscribed by transmembrane domains III, V, VI and VII. Again, this is in agreement with the crucial role played by the Phe8 side chain of AngII in the activation of AT1 receptor and the involvement of intrahelical amino acids in GPCR activation upon agonist binding (13). In the case of the AT2 receptor, there is no reported study (using either the structure-activity or the mutagenesis approach) on the crucial interaction leading to receptor activation. It is certainly related to the lack of a reliable functional assay for AT2 receptor. The information on the ligand binding domain of the AT2 receptor obtained with our photolabeling approach, will take its full significance only when a reliable functional assay will be available. The results described in this report represent only a minor part of the information needed to circumscribe to whole binding pocket of AngII receptors. Important information will be collected from further experiments with other photoligands bearing different photosensitive moieties at different positions. The same photoligands could also be used to photolabel the receptors under different conformational states (G-protein-coupled, uncoupled, constitutively active mutants, inactive mutants, etc.). This approach will provide useful information for the modeling of the three-dimensional structure of AngII receptors.
References
1. Timmermans, P.B., Duncia, J.V., Carini, D.J., Chiu, A.T., Wong, P.C., Wexler, R.R. and Smith, R.D. (1995) J. Hum. Hypertens. 9, Suppl. 5:S3-18.
2. deGasparo, M. and Levens, N. (1998) Pharmacol. Toxicol. 82 (6):257-271.
3. Guillemette, G. and Escher, E. (1983) Biochemistry 22 (24):5591-5596.
4. Guillemette, G., Guillon, G., Marie, J., Balestre, M.N., Escher, E. and Jard, S. (1986) Mol. Pharmacol. 30 (6):S544-551.
5. Servant, G., Boulay, G., Bossé, R., Escher, E. and Guillemette, G. (1993) Mol. Pharmacol. 43 (5):677-683.
6. Servant, G., Dudley, D.T., Escher, E. and Guillemette, G. (1994) Mol. Pharmacol. 45 (6):1112-1118.
7. Regoli, D., Park, W.K. and Rioux, F. (1974) Pharmacol. Rev. 26(2):69-123.
8. Karnik, S.S., Husain, A. and Graham, R.M. (1996) Clin. Exp. Pharmacol. Physiol. 3, Suppl.:S58-S66.
9. Hunyady, L, Balla, T. and Catt, K.J. (1996) Trends Pharmacol. Sci. 17 (4):135-140.
10. Noda, K., Saad, Y. and Karnik, S.S. (1995) J. Biol. Chem. 270 (48):28511-28514.
11. Feng, Y.H., Noda, K., Saad, Y., Liu, X.P., Husain, A. and Karnik, S.S. (1995) J. Biol. Chem. 270 (21):12846-12850.
12. Yamano, Y., Ohyama, K., Kikyo, M., Sano, T., Nakagomi, Y., Inoue, Y., Nakamura, N., Morishima, I., Guo, D.F., Hamakubo, T. et al. (1995) J. Biol. Chem. 270 (23):14024-14030.
13. Strader, C.D., Fong, T.M., Tota, M.R., Underwood, D. and Dixon, R.A. (1994) Annu. Rev. Biochem. 63:101-132.
| Discussion Board | Previous Page | Your Symposium |