Cardiovascular Diseases Poster Session
| INABIS '98 Home Page | Your Session | Symposia & Poster Sessions | Plenary Sessions | Exhibitors' Foyer | Personal Itinerary | New Search |
Introduction
Fluorescent probes for cell surface receptor molecules allow a high degree of visual and pharmacological analysis at the subcellular level in isolated tissues. Previous work by our group has demonstrated the fluorescent properties of QAPB for alpha1-adrenoceptors (McGrath et al 1996, Daly et al 1998) establishing the method of fluorescent ligand binding (at nanomolar concentrations) and its application for the study of receptors in single ‘living’ cells (Mackenzie et al 1998). It was shown that specific fluorescent-ligand binding could be assessed on individual cells. This fluorescent ligand allows visualisation with high resolution of binding sites on the surface of live cells and hence of their distribution within heterogeneous tissues. Fluorescence is easily quantified using confocal microscopy (McGrath et al 1996). The quinazoline confers the compound’s lipohilic properties and hence spatial distribution of receptors throughout the cell is apparent at nanomolar concentrations of the ligand on recombinant receptors.
We have now extended the scope of this method to allow identification of native alpha1-adrenoceptors by examining the fluorescent binding of QAPB in rat cerebral basilar smooth muscle cells. A report by Hogestatt and Andersson in 1984 showed regional differences of alpha adrenoceptors in the rat cerebral vessels. However, an earlier report suggested that rat anterior and basilar arteries do not contain classical alpha adrenoceptors (Silverberg et al 1982). We believe that this method of analysis will allow clear distinction and clarification of alpha adrenoceptors from native smooth muscle cells dissociated from any vascular bed or non-vascular tissue.
Materials and Methods
Primary Smooth muscle cell dissociation.
Cells were dissociated by the method of Kamishima et al 1997. Briefly, rat basilar arteries were dissected and immediately placed in buffer 1 (147mM NaCl, 5mM KCl, 1mM MgCl2, 1.8mM CaCl2, 10mM HEPES, 0.1% BSA, pH7.4). Arteries were washed once in buffer 1, resuspended in buffer 2 (80mM sodium glutamate, 54mM NaCl, 5mM KCl, 1mM MgCl2, 0.1mM CaCl2, 10mM HEPES, 10mM glucose, 0.2mM EDTA, 0.1% BSA, pH7.3) with 1.7mM papain, 0.7mM dithioerythritol and incubated at 35°C for 30mins. Arteries were centrifuged at 1200g for 2mins and supernatant discarded. Arteries were resuspended in buffer 2 with 1.0mM collagenase II, 1.0mM hyaluronidase and SMC were dispersed immediately with a fire polished pasteur pipette. Cells were plated onto coverslips.
Fluorescent binding studies.Dissociated SMC were mounted in a flow chamber and cells were washed three times with HEPES buffer (130 mM NaCl, 5mM KCl, 20mM HEPES, 10mM Glucose, 1mM MgCl2, 1mM CaCl2). After baseline was achieved the first concentration of fluorescent ligand, QAPB was added and allowed to equilibrate for at least 5 minutes. Cumulative concentrations (0.4-10nM) of QAPB were added to the bath and non-specific binding was defined as binding in the presence of 10mM phentolamine.
Whole cell image analysis.Images were collected using an invert (Nikon Diaphot) microscope fitted with a Noran Odyssey Laser Scanning Confocal Module. The 488nm line (515 band pass) of an argon-ion laser was used throughout. Using cell autofluorescence a suitable group of smooth muscle cells were selected and the focal plane fixed by locking the focus motor. The system was then set to acquire images (64 frame averages; 2.56 seconds exposure) at 1 minute intervals. Universal Imaging's 'MetaMorph' software define-region tool was used to select the cell area and intensity was measured over time for each cell. Binding isotherms were fitted to equilibrium fluorescence data using GRAPHPAD Prism software to determine the fluorescent KD.
3D Image AnalysisSerial Z-sections of QAPB (10nM) binding to rat cerebral SMC were acquired at 0.25µm steps with a 15µm pinhole. 3D images were constructed using the 3D-iso-surface module in IMARIS on a SGI workstation. The images were initially processed in ‘Metamorph’ by low pass filtering. This was followed by multi-thresholding to segment the image volume and distance transform applied to create concentric shells of the cell. The intensity mapped onto each shell was then plotted against axial depth to provide a graph of intensity versus depth.
Results
Fluorescence binding was time and concentration dependent in rat basilar smooth muscle cells (figure 1).
 Figure 1. Smooth muscle cells were dissociated from rat cerebral artery, plated on coverslips and examined by confocal microscopy with time-lapse photography at 1min intervals in the presence of increasing concentrations of QAPB (Ex 488nm/Em 515nm). Figures 1a and 2a show bright field images of smooth muscle cells prior to the start of the experiment. Figures 1b-1g and 2b-2g show images collected at equilibrium time points for each concentration of QAPB (added at 5min intervals) in absence and presence of 10uM phentolamine respectively; 0, autofluoresence; 0.4nM; 1nM; 2nM; 5nM; 10nM. Images are representative of two experiments. Images are shown in pseudocolour, where black indicates no staining and blue, green, yellow and red indicate increasing levels of saturation of the fluorophore.
Figure 1. Smooth muscle cells were dissociated from rat cerebral artery, plated on coverslips and examined by confocal microscopy with time-lapse photography at 1min intervals in the presence of increasing concentrations of QAPB (Ex 488nm/Em 515nm). Figures 1a and 2a show bright field images of smooth muscle cells prior to the start of the experiment. Figures 1b-1g and 2b-2g show images collected at equilibrium time points for each concentration of QAPB (added at 5min intervals) in absence and presence of 10uM phentolamine respectively; 0, autofluoresence; 0.4nM; 1nM; 2nM; 5nM; 10nM. Images are representative of two experiments. Images are shown in pseudocolour, where black indicates no staining and blue, green, yellow and red indicate increasing levels of saturation of the fluorophore.
In the presence of phentolamine (10uM) QAPB binding was significantly inhibited and the remaining binding was used to define non-specific binding. A specific binding curve was constructed and a fluorescent KD value calculated as 1.13nM (figure 2).
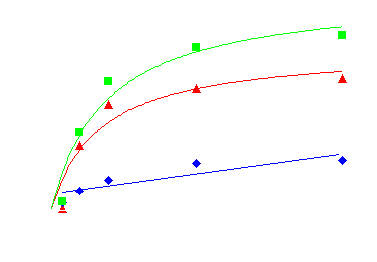 Figure 2. Binding of fluorescent labelled ‘prazosin’ (QAPB) to alpha1-adrenoceptors. Non-specific binding (blue) was determined in the presence of 10uM phentolamine. Specific binding (red) was calculated as total binding (green) minus non-specific binding. Scatchard analysis of the specific binding data produced a fluorescent KD value of 1.13nM.
Figure 2. Binding of fluorescent labelled ‘prazosin’ (QAPB) to alpha1-adrenoceptors. Non-specific binding (blue) was determined in the presence of 10uM phentolamine. Specific binding (red) was calculated as total binding (green) minus non-specific binding. Scatchard analysis of the specific binding data produced a fluorescent KD value of 1.13nM.
.
The cellular distribution was shown to be closely associated with both the plasma membrane and the nuclear membrane (figure 3).
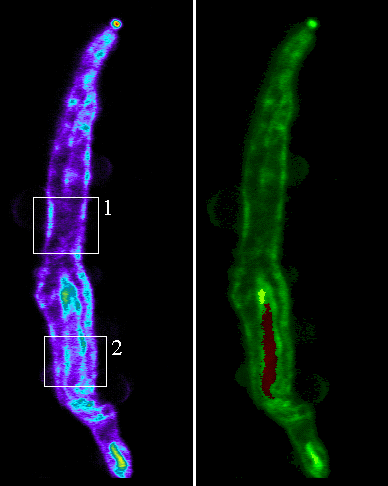 Figure 3. Smooth muscle cells were dissociated from the rat cerebral artery and plated onto coverslips. Serial sections were acquired at 0.25&mirco;m steps with a confocal laser scanning microscope. The images above show longitudinal sections through the cell. The area in box 1 relates to figure 2 and the area in box 2 relates to figure 3. The cell shown in green showns the location of QAPB in relation to the nucleus in red.
Figure 3. Smooth muscle cells were dissociated from the rat cerebral artery and plated onto coverslips. Serial sections were acquired at 0.25&mirco;m steps with a confocal laser scanning microscope. The images above show longitudinal sections through the cell. The area in box 1 relates to figure 2 and the area in box 2 relates to figure 3. The cell shown in green showns the location of QAPB in relation to the nucleus in red.
In addition the fluorescent compound revealed two distinct populations of diffuse and clustered binding sites. The diffuse component was evident up to a concentration of 5nM QAPB whereas the clustered component was found to be mainly located intracellularly.
When the images of cells were viewed using quantitative assessment of the sub-cellular localisation of binding, there was a high level of intracellular binding. Therefore, we devised a new quantitative method for analysis of fluorescence in 3D so that it was possible to distinguish receptors on the cell surface from those inside the cells. The fluorescence intensity was plotted against axial depth and is shown in figure 4.
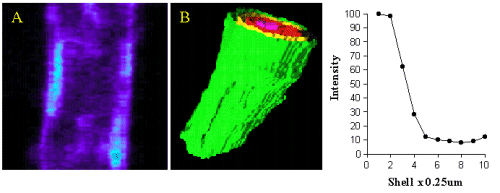 Figure 4. Image A shows QAPB (10nM) binding to an area of plasma membrane on a smooth muscle cell. The light blue area represents clustered binding to alpha1-adrenoceptors on the membrane. An iso-shell model was created from Image A and is shown as Image B. Concentric rings are shown as green (membrane), yellow, red and pink as they reach the inner core of the cell. The graph depicts the relationship between intensity of alpha1-adrenoceptor binding to depth with in the cell starting at the plasma membrane.
Figure 4. Image A shows QAPB (10nM) binding to an area of plasma membrane on a smooth muscle cell. The light blue area represents clustered binding to alpha1-adrenoceptors on the membrane. An iso-shell model was created from Image A and is shown as Image B. Concentric rings are shown as green (membrane), yellow, red and pink as they reach the inner core of the cell. The graph depicts the relationship between intensity of alpha1-adrenoceptor binding to depth with in the cell starting at the plasma membrane.
Most of the binding was located in the outer shell (green) on the plasma membrane and in figure 5 (red)on the nuclear membrane.
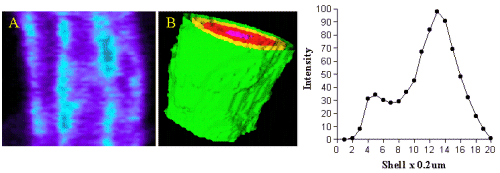 Figure 5. Image A shows QAPB (10nM) binding to an area of both the plasma membrane and nuclear membrane on a smooth muscle cell. The light blue area represents clustered binding to alpha1-adrenoceptors on both the plasma and nuclear membranes. An iso-shell model was created from Image A and is shown as Image B. Concentric rings are shown as green (membrane), yellow, red and pink as they reach the inner core of the cell. The graph depicts the relationship between intensity of alpha1-adrenoceptor binding to depth with in the cell starting at the membrane.
Figure 5. Image A shows QAPB (10nM) binding to an area of both the plasma membrane and nuclear membrane on a smooth muscle cell. The light blue area represents clustered binding to alpha1-adrenoceptors on both the plasma and nuclear membranes. An iso-shell model was created from Image A and is shown as Image B. Concentric rings are shown as green (membrane), yellow, red and pink as they reach the inner core of the cell. The graph depicts the relationship between intensity of alpha1-adrenoceptor binding to depth with in the cell starting at the membrane.
Intensity of binding reduced from the outer shell towards the inner shell (nucleus). Since the nucleus does not have any binding at all the intensity value in the centre of the cell was very low in comparison to the membrane.
Discussion and Conclusion
A report recently highlighted the differences in subcellular location of the alpha1-adrenoceptors in recombinant cells where the a1a-receptor appears to be predominantly intracellular whereas the a1b-receptor associates mainly with the surface membrane (Hirisawa et al 1997). We have shown intracellular binding sites in rat basilar smooth muscle cells, particularly around the nucleus, which could represent binding in the Golgi. We have found that QAPB will enter the cells slowly at low concentrations (<5nM) and rapidly at high concentrations (>10nM). This property of QAPB enables the ligand to bind both surface and intracellular sites if sufficient time is given to reach equilibrium. The intracellular binding sites are presumably not involved in transduction of transmembrane signals but represent newly synthesized or recycling stores of receptors.
Concentration dependent binding of QAPB was detected in smooth muscle cells dissociated from rat basilar arteries. Binding isotherms were calculated from specific binding data and revealed a fluorescent KD of 1.13nM which is similar to results obtained from fluorescent experiments performed on recombinant cells (Mackenzie et al 1998). This method demonstrates that single live native cells can have specific binding sites for this fluorescent probe. This is in contrast to an earlier report by our group where minimal binding of QAPB was observed in the rat basilar artery (McGrath et al 1996). However, the level of binding intensity seen in basilar smooth muscle cells was not high compared to binding of QAPB in recombinant cells suggesting that the Bmax may be much reduced in these vessels. This would correspond with reports of low specific binding to rat basilar artery membranes (Silverberg et al 1982). However, the images shown and differences in subcellular location of alpha adrenoceptors highlights the importance of examining ‘live’ cells as opposed to crude membrane fractions since the majority of QAPB binding was found to be intracellular.
References
McGrath JC, Arribas SM & Daly CJ (1996). The use of fluorescent ligands for the study of receptors. T.i.P.S; 17 (11):393-399.
Daly CJ, Milligan CM, Milligan G, Mackenzie JF & McGrath JC (1998). Cellular localisation and pharmacological characterization of functioning alpha1-adrenoceptors by fluorescent ligand binding and image analysis reveals identical binding properties of clustered and diffuse populations of receptors. J. Pharmacol. Exp. Ther. 286; 984-990.
Mackenzie JF, Daly CJ & McGrath JC (1998). Comparison and validation of human alpha1-adrenoceptor binding characteristics on live, whole cells using radioligand binding and confocal laser scanning microscopic techniques. Nauyn-Schmiedeberg’s Arch. Pharmacol., 358; suppl. 2 , p6.45.
Hogestatt ED, Andersson K-E. (1984). On the post-junctional alpha-adrenoceptors in rat cerebral and mesenteric arteries. J. Aut. Pharmacol. 4(3); 161-173.
Silverberg GD, Neild TO, Adams A, Jarrott B (1982). Lack of alpha-adrenoceptor binding of [125I]BE2254 to rat basilar artery membranes. Neuroscience Letters 32 (2), 109-111.
Kamishima, T & McCarron, J.G. (1997) Regulation of the cytosolic Ca2+ concentration by Ca2+ stores in single smooth muscle cells from rat cerebral arteries. Journal of Physiology Vol 501 (3); 497-508.
Hirisawa A, Sugawara T, Awaji T, Tsumaya K, Ito H & Tsujimoto G (1997). Subtype-specific differences in subcellular localisation of alpha1-adrenoceptors: Chloroethylclonidine preferentially alkylates the accessible cell surface alpha1-adrenoceptors irrespective of the subtype. Mol. Pharmacol., 52, 764-770.
| Discussion Board | Previous Page | Your Poster Session |