Cell Biology Poster Session
| INABIS '98 Home Page | Your Session | Symposia & Poster Sessions | Plenary Sessions | Exhibitors' Foyer | Personal Itinerary | New Search |
Introduction
The acrosome reaction, an exocytotic process, is an essential step for mammalian spermatozoa to be able of fertilizing homologous oocytes (Yunes et al., 1992; Barros et al., 1993a, 1993b; Valdivia et al., 1994). The presence of heterotrimeric G-proteins in invertebrate and mammalian spermatozoa has been reported by different groups (Kopf et al., 1986; Garty et al., 1988; Glassner et al., 1991; Hinsch et al., 1992). Furthermore, several reports have shown triggering of the acrosome reaction in mammalian spermatozoa by activation of G proteins (Endo et al., 1987; Domínguez et al., 1995, 1996). Like many exocytotic events in somatic cells, the acrosome reaction is regulated, upon activation of one or more G-proteins, by several second messenger systems (Lee et al., 1992; Tesarik et al., 1993; Ward et al., 1992). However, and notwithstanding the important advances made during the past years on the knowledge of the signalling mechanims involved on the acrosome reaction (AR), the study of this exocytotic process on mammalian spermatozoa is seriously limited by the fact that putative important macromolecules -macromolecules that are known to be present inside the cell- are completely unable of entering the cell. In order to avoid this technical limitation we developed and tested a method of permeabilization of human spermatozoa with streptolysin-O (SLO).
Materials and Methods
Reagents
Calcium ionophore A23187, GTPgS, GDPbS, lisophosphatidylcholine, mastoparan, fluorescein isothiocyanate-labelled Pisum sativum lectin (FITC-PSA), were obtained from Sigma Chemical Company (St. Louis, MO). [AlF4]- was prepared from original stocks. Streptolysine-O was obtained from Welcome Dagnostics. The culture media was GPM (Gamete Preparation Media, Serono-Spain).Water for all solutions was purified with a batch-fed system (Barnsted EASYpure, Dubuque, IA).
Experimental design
Human semen samples from at least 5 healthy donors of proven fertility (motility >50%, motile spermatozoa >60x106/ml) were used. After swim-up separation (Makler et al., 1984) for 1 h at 37ºC in an atmosphere of 95% air - 5% CO2 using GPM culture media, highly motile sperm were recovered. The concentration was adjusted to 1-5x106/ml in fresh culture media, and incubation proceeded for additional 2 h at 37ºC and 5% CO2, accounting for a total incubation time of 3 h under capacitating conditions. In different sets of experiments, the following reagents were tested: 1) vehicle (control groups); 2) calcium (0.4-0.7 mM); 3) calcium ionophore A23187 (5 microM); 4) lisophosphatidylcholine (LPC, 25 microM); 5) GTPgS (40 microM); 6) GDPbS (200 microM); 7) [AlF4]- (5 mM NaF and 100 microM Al2(SO4)3); and 8) mastoparan (MP, 20 microM). Permeabilization was performed at 4ºC after washing the incubated samples with cold PBS for two times, by adding SLO (0.5 UI/ml final concentration). After 15 min the sample was washed again with cold PBS, and finally resuspended in sucrose-buffer with DTT 2.0 mM. Temperature was slowly increased to 37ºC and, after 15 min, all reagents were added to 100 microliters aliquots for 15 additional min. Non permeabilized samples were treated in a similar way, except for the fact they were not permeabilized.
Acrosome reaction assay
The acrosome reaction was evaluated by the FITC-PSA lectin according to Mendoza et al. (1992). Briefly, the cell suspensions were centrifuged for 1 min in a Beckman Microfuge (at least two cycles) and the pellets resuspended in phosphate buffered saline (PBS) for 3 min. A 10 ml drop was then placed on a spotted-slide and air-dried at room temperature. After air-drying, the sperm smears were fixed with cold (4ºC) methanol for 30 sec and incubated in the darkness with 50 mg/ml FITC-PSA in PBS in a moisturized chamber for 30 min at room temperature. After intensive washing with distilled water, unmounted slides were examined on the same day of the staining procedure, and at least 200 live cells were evaluated in a epifluorescence Nikon Optiphot II microscope (100x) according to the following patterns: a) selective staining of the whole acrosome (unreacted cell); b) no staining at all, or staining limited to the equatorial segment (reacted cells).
Statistical analysis
Differences between experimental and control conditions were tested by One-Way ANOVA and Fisher's PSLD tests. Where necessary, percentages were transformed to the arc-sine prior to analysis. Significant differences were those where p was <0.05
Results
Almost all spermatozoa resulted permeabilized since most of them (>99%) were stained by the supravital stain eosin-Y, indicating that the cells were functionally unable of maintaining the stain outside the intracellular space (by definition we could consider the cells functionally dead). We found the non-permeabilized cells to be able of responding to A23187, indicating the acrosomes were functionally competents. Also, when we treated the cells with calcium, we could see that calcium (0.5 mM was significantly different from controls and calcium 0.4 mM, with a plateau at 0.6 mM) increased AR to levels comparable to those reached by intact spermatozoa treated with well known stimulants like LPC and A23187, as can be seen in Figure 1.
 Fig. 1: Percentage of acrosome
reaction in non-permeabilized and permeabilized spermatozoa. Iono= calcium
ionophore A23187; CaCl2= calcium. Different letters indicate significant
differences p < .05
Fig. 1: Percentage of acrosome
reaction in non-permeabilized and permeabilized spermatozoa. Iono= calcium
ionophore A23187; CaCl2= calcium. Different letters indicate significant
differences p < .05
Also, G-protein stimulation with mastoparan, GTPgS or AlF increased AR. On the other hand, GDPbS, a G-protein inhibitor, was able of blocking the increase of AR induced by calcium, as can be seen in Figure 2.
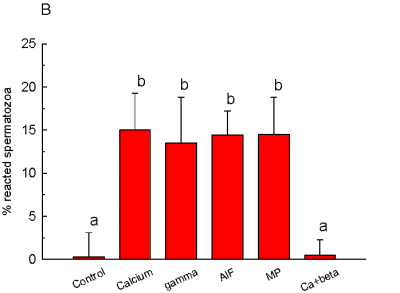 Fig. 2: Percentage of acrosome
reaction in permeabilized spermatozoa. gamma= GTPgS; AlF= [AlF4]; MP= mastoparan;
Ca+beta= calcium + GDPbS. When two reagents are mentioned, the first one
was added 15 min before the second one. Different letters indicate significant
differences p < .05
Fig. 2: Percentage of acrosome
reaction in permeabilized spermatozoa. gamma= GTPgS; AlF= [AlF4]; MP= mastoparan;
Ca+beta= calcium + GDPbS. When two reagents are mentioned, the first one
was added 15 min before the second one. Different letters indicate significant
differences p < .05
Discussion and Conclusion
Our results suggest that permeabilization of human spermatozoa with SLO does not affect the intrinsic capability of the acrosome to respond to different stimulus, notwithstanding the fact that all spermatozoa could be considered technically dead. In fact, we consider the whole process to be far from physiologically acceptable. The fact is, however, that we do not wanted to obtain a physiologically valid model but one eventually useful to test macromolecules during the cascade of signals conducting to the acrosome reaction. Also, from our data, we reinforce the idea of both a G-protein and calcium participating during the AR.
It is concluded that the model could be eventually useful to study macromolecules involved on the acrosome reaction of human spermatozoa.
References
- Barros C, Meléndez J, Valdivia M, Ríos M, Yunes R (1993a): Sperm passage through the egg coats. Biol Research 26:417-427.
- Barros C, Meléndez J, Valdivia M, Yunes R, Ríos M (1993b): Protease involvement in penetration of egg coats: A comparative approach. J Reprod Dev 39:71-72.
- Domínguez L, Díaz A, Fornés MW, Mayorga LS (1995): Reagents that activate GTP-binding proteins trigger the acrosome reaction in human spermatozoa. Int J Androl 18:203-207.
- Domínguez L, Yunes R, Fornés MW, Mayorga L (1996): Acrosome reaction stimulated by the GTP non-hydrolizable analogue GTPgS is blocked by phospholipase A2 inhibitors in human spermatozoa. Int J Androl 19:248-252.
- Endo Y, Lee MA, Kopf GS (1987): Evidence for the role of a guanine nucleotide-binding regulatory protein in the zona pellucida-induced mouse sperm acrosome reaction. Dev Biol 119:210-216.
- Garty NB, Galiani D, Aharonheim A, Ho Y, Phillips DM, Dekel N, Salomon Y (1988): G-proteins in mammalian gametes:an immunocytochemical study. J Cell Sci 91:21-31.
- Glassner M, Jones J, Kligman I, Woolkalis MJ, Gerton GL, Kopf GS (1991): Immunocytochemical and biochemical characterization of nucleotide-binding proteins in mammalian spermatozoa. Dev Biol 146:438-450.
- Hinsch KD, Hinsch E, Aumüller G, Tychowiecka I, Schultz G, Schill WB (1992): Immunological identification of G-protein a and b subunits in tail membranes of bovine spermatozoa. Biol Reprod 47:337-346.
- Kopf GS, Woolkalis MJ, Gerton GL (1986): Evidence for a guanine nucleotide-binding regulatory protein in invertebrate and mammalian sperm. J Biol Chem 261:7327-7331.
- Lee MA, Check JH, Kopf GS (1992): A guanine nucleotide-binding regulatory protein in human sperm mediates acrosomal exocytosis induced by the human zona pellucida. Mol Reprod Dev 31:78-86.
- Makler A, Murillo O, Huszar G, Tarlatzis B, DeCherney A, Naftolin F (1984): Improved techniques for collecting motile spermatozoa from human semen. I. A self-migratory method. Int J Androl 7:61-70.
- Mendoza C, Carreras A, Moos J, Tesarik J (1992): Distinction between true acrosome reaction and degenerative acrosome loss by a one-step staining method using Pisum sativum agglutinin. J Reprod Fertil 95:755-763.
- Tesarik J, Carreras A, Mendoza C (1993): Differential sensitivity of progesterone-induced and zona pellucida-induced acrosome reactions to pertussis toxin. Mol Reprod Dev 34:183-189.
- Ward CR, Storey BT, Kopf GS (1992): Activation of a Gi protein in mouse sperm membranes by solubilized proteins of the zona pellucida, the egg's extracellular matrix. J Biol Chem 267:14061-14067.
- Valdivia M, Yunes R, Meléndez J, De Ioannes A, Leyton L, Becker MI, Barros C (1994): Immunolocalization of proacrosin/acrosin in rabbit sperm during acrosome reaction and in spermatozoa recovered from the perivitelline space. Mol Reprod Dev 37:216-222.
- Yunes R, Meléndez J, Valdivia M, Barros C (1992): Golden hamster perivitelline spermatozoa do not show proacrosin/acrosin at the inner acrosomal membrane. Biol Res 25:91-93.
| Discussion Board | Previous Page | Your Poster Session |