Cell Biology Poster Session
| INABIS '98 Home Page | Your Session | Symposia & Poster Sessions | Plenary Sessions | Exhibitors' Foyer | Personal Itinerary | New Search |
Introduction
Calmodulin (CaM) is a ubiquitous calcium binding protein with a molecular weight of approximately 17kD. X-ray crystallographic studies have revealed that CaM is an elongated, dumbbell-shaped molecule with two globular domains, each containing two Ca2+-binding sites. The helix joining the two ends has been proposed to undergo length changes upon calcium binding. Other studies have also demonstrated that the conformation of CaM is pH sensitive, which exists in a relatively compact conformation at neutral pH's, but becomes more elongated at pH 5. Enzymatic data indicate substantially lower activation of phosphodiesterase from unheated calmodulin (10.9 nmol/mg min) than from heated calmodulin (20.2 nmol/mg min). Heat treatment is omitted in the purification of unheated calmodulin. (See Materials and Methods) However, heat treatment on purified unheated calmodulin increases its stimulatory activity on phosphodiesterase. These data suggest the involvement of a possible heat-labile regulatory molecule and associated conformational changes in the activity as well as regulation of calmodulin. It has also been suggested that calmodulin aggregate under certain conditions. The goal of this study is to demonstrate the existence of aggregation in calmodulin under different conditions through high performance liquid chromatography. The effects of heat-induced structural changes will also be investigated using electrophoresis under non-denaturing and denaturing conditions.
Materials and Methods
Calmodulin Purification Calmodulin (heated) was isolated from bovine testes purchased from a local slaughter house. A 20% (w/v) bovine testes homogenate was centrifuged at 25,000g for 1 hr. The resulting supernatant was brought to a rapid boil and centrifuged at 25,000g for 30 min. The supernatant was made 70% saturated with ammonium sulfate and centrifuged at 25,000g for 30 min. The resulting pellet was dissolved in 15 mL of Tris buffer, pH 7.5 and applied onto a phenothiazine-affinity column. The purified sample was lyophilized and stored at -20oC. The heat treatment was omitted to obtain unheated calmodulin. The same samples were used in subsequent enzymatic, chromatographic and electrophoretic studies.
Gel Electrophoresis
A NOVEX mini-gel system with pre-cast gels were used:
Denaturing: NOVEX 4-12% Bis-Tris Gel with MES-SDS running buffer.
Non-denaturing: NOVEX 8-16% Tris-Glycine Gel with Tris-Glycine running buffer.
High Performance Liquid Chromatography
All HPLC experiments were performed on commercially available HPLC instruments: Spectra-Physics 8800 HPLC equipped with a 10 uL sample loop and a ternary pump with a spectra-Physics 8480XR scanning detector set at 280 and a Spectra-Physics 4270 integrator. 10uL of 20 ug/uL calmodulin samples were injected onto a Supelco TSK-Gel SW3000XL gel permeation column. Buffers ranging from pH 4.5 to 7.5 at a flow rate of 1 mL/min were used as the mobile phase.
Results
Gel Electrophoresis
With only one band obtained from each calmodulin sample as can be seen in Figure 1a, SDS-PAGE supports the purity of both the heated and unheated calmodulin samples used. Figure 1b shows the separation of calmodulin samples in the non-denaturing gel, which indicates the presence of a high molecular weight aggregate in the unheated samples, although it was not present in a large concentration.
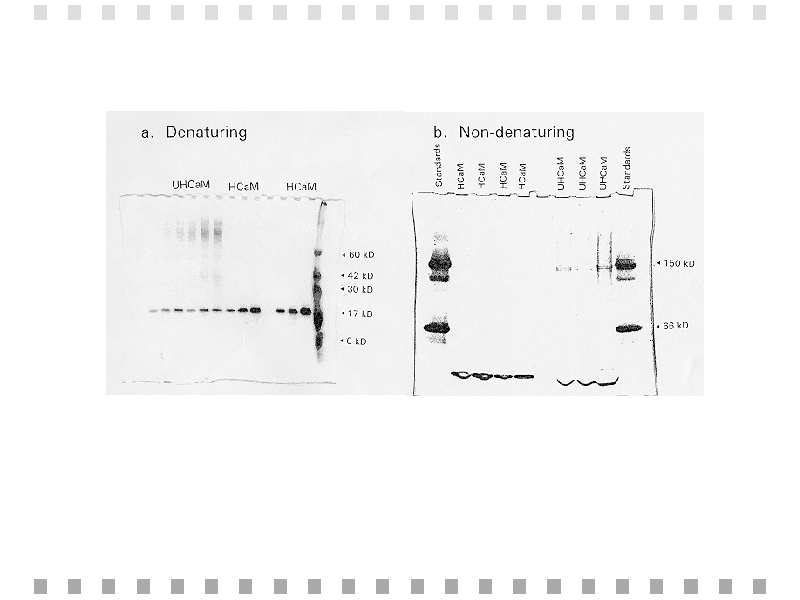 Fig. 1: Denaturing (a) and non-denaturing (b) gel electrophoresis results at various concentrations of calmodulin. A faint band in the non-denaturing gel indicates the presence of a 150 kD aggregate.,BR CLEAR="ALL">
Fig. 1: Denaturing (a) and non-denaturing (b) gel electrophoresis results at various concentrations of calmodulin. A faint band in the non-denaturing gel indicates the presence of a 150 kD aggregate.,BR CLEAR="ALL">
HPLC Chromatograms
The retention times for the monomer and the aggregate of interest were determined to be ~12 sec and ~10 sec, respectively. The monomer to aggregate ratio (M/A) was calculated using the peak areas of the monomer and the aggregate.
Heated Calmodulin (HCaM)
Heated calmodulin samples were separated by HPLC at various pH values and a flow rate of 1 mL/min as can be seen in Figure 2. To aid in comparison, the peak areas and the monomer-to-aggregate ratios were plotted as a function of pH, which is shown in figure 3.
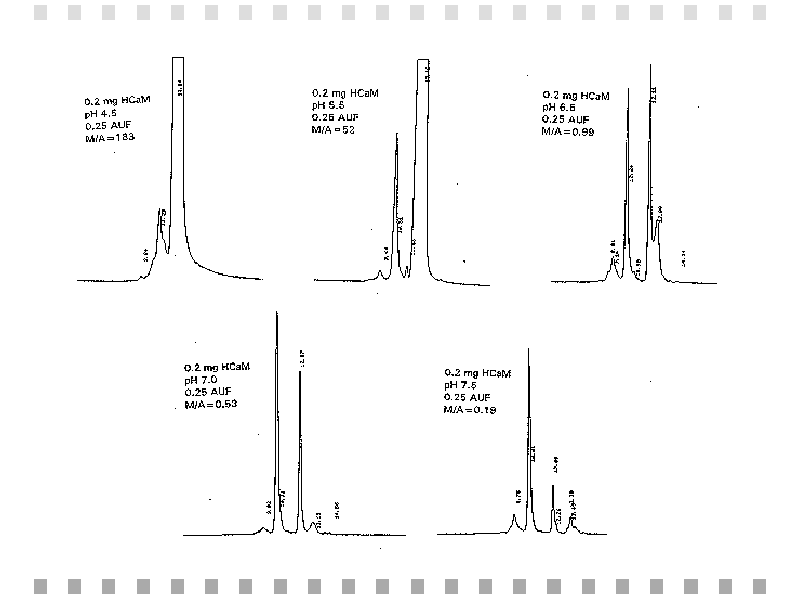 Fig. 2: Gel permeation HPLC chromatograms of 0.2 mg of heated calmodulin (HCaM) at various pH values and a flow rate of 1 mL/min.,BR CLEAR="ALL">
Fig. 2: Gel permeation HPLC chromatograms of 0.2 mg of heated calmodulin (HCaM) at various pH values and a flow rate of 1 mL/min.,BR CLEAR="ALL">
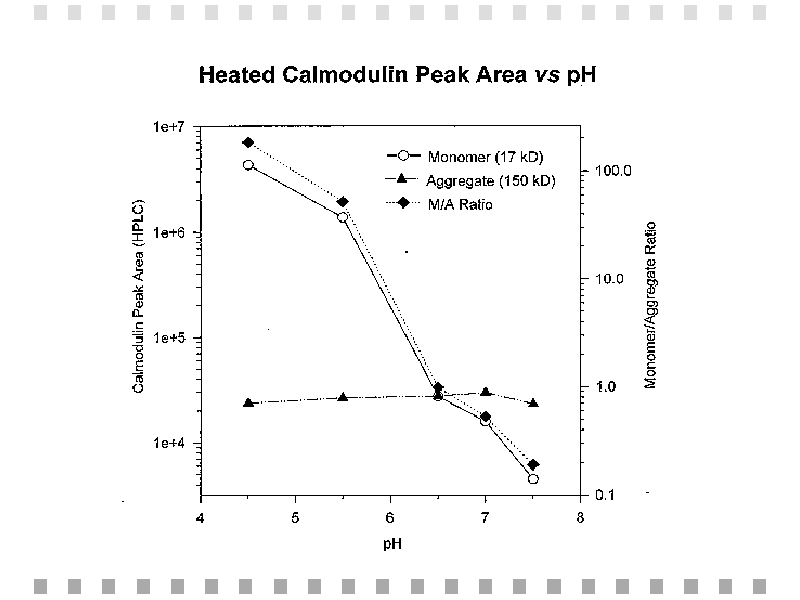 Fig. 3: Heated calmodulin peak areas obtained from gel permeation HPLC and monomer to aggregate ratios (M/A) are plotted against pH on a log scale.,BR CLEAR="ALL">
Fig. 3: Heated calmodulin peak areas obtained from gel permeation HPLC and monomer to aggregate ratios (M/A) are plotted against pH on a log scale.,BR CLEAR="ALL">
Unheated Calmodulin (HCaM)
Chromatographic results obtained from unheated calmodulin samples were at various pH values are summarized in Figure 4. Figure 5 shows the relationship between peak areas and monomer-to-aggregate ratios with pH.
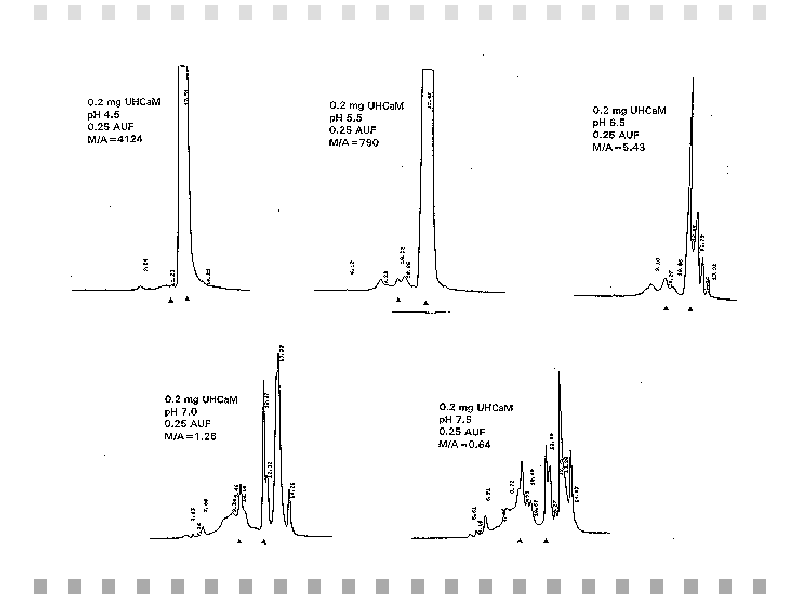 Fig. 4: Gel permeation HPLC chromatograms of 0.2 mg of unheated calmodulin (UHCaM) at various pH values and a flow rate of 1 mL/min.,BR CLEAR="ALL">
Fig. 4: Gel permeation HPLC chromatograms of 0.2 mg of unheated calmodulin (UHCaM) at various pH values and a flow rate of 1 mL/min.,BR CLEAR="ALL">
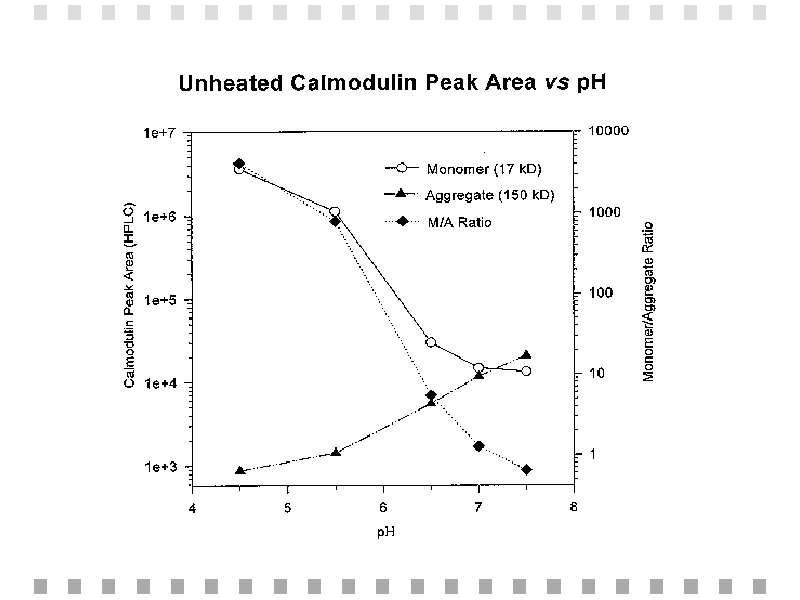 Fig. 5: Unheated calmodulin peak areas obtained from gel permeation HPLC and monomer to aggregate ratios (M/A) are plotted against pH on a log scale.,BR CLEAR="ALL">
Fig. 5: Unheated calmodulin peak areas obtained from gel permeation HPLC and monomer to aggregate ratios (M/A) are plotted against pH on a log scale.,BR CLEAR="ALL">
Discussion and Conclusion
Electrophoretic data demonstrated evident aggregation in the unheated calmodulin. However, the HPLC results indicate that both heated and unheated calmodulin form an aggregate and aggregation is a function of pH. Furthermore, aggregation appeared to be more pronounced in the heated calmodulin (see Fig. 2). In addition to the aggregate of focus in this study (150 kD), the unheated calmodulin also forms aggregates of other molecular weights (see Fig. 4). This may result in a decrease in the net amount of "functional" calmodulin, leading to minimized enzymatic activation in the previous studies.
Aggregation may be involved in controlling calcium/calmodulin-modulated metabolism, i.e. calmodulin activity may be regulated by a disturbed equilibrium between monomer and aggregate as a function of subcellular local calcium concentrations and pH.
References
1. C.B. Klee, T.H. Crouch & P.G. Richman (1980) Ann. Rev. Biochem. 49: 489-515.
2. Y. S. Babu, J.S. Sack, T.J. Greenhough, C.E. Bugg, A.R. Means & W.J. Cook (1985) Nature 315:37-40.
3. Y. Yao, C. Schoneich & T.C. Squier (1994) Biochemistry 33:7797-7810
4. C.-L. Wang (1989) Biochemistry 28:4816-4820
5. J. Trewhella, W.K. Liddle, D.B. Heidorn & N. Strynadka (1989) Biochemistry 28:1294-1301
| Discussion Board | Previous Page | Your Poster Session |