Cell Biology Poster Session
| INABIS '98 Home Page | Your Session | Symposia & Poster Sessions | Plenary Sessions | Exhibitors' Foyer | Personal Itinerary | New Search |
Results
TABLE I
Total PKA Activity and Effect of Hibernation on the Percentage of PKA Present as the Free Catalytic Subunit in Bat Tissues
Tissue Total Activity % PKAc
Control Hibernation
Skeletal Muscle 4.5 ± 0.6 35.4 ± 2.7 52.0 ± 1.9a
Liver 21.6 ± 4.0 14.8 ± 0.8 11.7 ± 0.7
Thymus 29.5 ± 2.2 36.6 ± 3.2 18.8 ± 0.6a
Spleen 28.0 ± 3.2 89.2 ± 3.0 43.1 ± 2.6a
White Adipose 17.3 ± 1.0 30.0 ± 2.5 47.0 ± 4.8a
Brown Adipose 41.2 ± 3.7 11.9 ± 0.6 12.1 ± 0.4
Brain 32.9 ± 4.0 37.4 ± 5.1 36.1 ± 4.6
Heart 12.6 ± 1.1 10.4 ± 1.1 7.3 ± 0.7
Kidney 16.7 ± 1.2 16.5 ± 1.1 14.7 ± 0.5
Data are means ± SEM, n = 3. Total PKA activity in tissues from euthermic, control bats is reported as units/gram wet weight at 22°C; total activity in hibernator tissues was not significantly different in any instance. a Significantly different from the corresponding control value using the Student's t-test, p<0.05. TABLE II
Purification of Bat Skeletal Muscle PKA Free Catalytic Subunit
Purification Total Total % Yield Fold Specific
Step Protein Activity Purif. Activity
(mg) (units) (units/mg)
Crude 24.72 11.51 -- -- 0.466
DE-52 cellulose 10.11 280.5 100 59.5 27.7
Hydroxylapatite 2.63 328.8 100 268 125.0
Prot. agarose 1.18 170.1 52 310 144.4
Blue dextran 0.48 98.3 30 439 204.7
Enzyme assays conducted at 22°C.
TABLE IIISubstrate Affinity Constants and I50 Values for Purified Bat Skeletal Muscle PKAc and Commercial Porcine Heart PKAc at Two Assay Temperatures
Bat Muscle Porcine Heart
37°C 5°C 37°C 5°C
Km (µM)
Kemptide 9.1 ± 0.2 3.4 ± 0.1a 11.9 ± 1.2 2.2 ± 0.03a
Mg-ATP 94.1 ± 4.5 49.2 ± 4.5a 25.0 ± 1.2 14.7 ± 0.9a
I50 (mM)
NaCl 461 ± 2.6 487 ± 13.7 388 ± 3.9 461 ± 13.3a
KCl 456 ± 8.8 582 ± 26.8a 378 ± 11.8 606 ± 10.7a
NH4CL 349 ± 13.5 347 ± 22.8 232 ± 0.9 410 ± 9.1a
(NH4)2SO4 108 ± 2.0 196 ± 8.6a 74.9 ± 4.0 186 ± 2.4a
NaF 37.8 ± 0.8 38.4 ± 1.1 38.9 ± 0.5 11.4 ± 0.7a
Data are means ± SEM, n = 3. aSignificantly different from corresponding value at 37°C using the Student's t-test, p< 0.05.
TABLE IVProtein Kinase Inhibitors of Bat Skeletal Muscle PKAc
Inhibitor Concentration Activity % Inhibition Protein Target
Control 205 0
PKAi 0.1 µM 118 42 PKA 2.0 µM 18 91
H-89 0.05 µM 150 27 PKA 1.0 µM 55 73
Calphostin C 0.1 µM 86 58 PKC 20.0 µM 77 62
PKCi 0.05 µM 168 17 PKC 1.0 µM 182 11
Lav A 1.0 nM 173 15 TPK 20.0 nM 164 21
KT-5823 0.1 µM 168 18 PKG 20.0 µM 150 27
Data are n = 1. Activities are units/mg protein at 22°C. PKC is calcium/ phospholipid dependent protein kinase, TPK is tyrosine protein kinase, and PKG is 3'-5'-cyclic GMP dependent protein kinase.
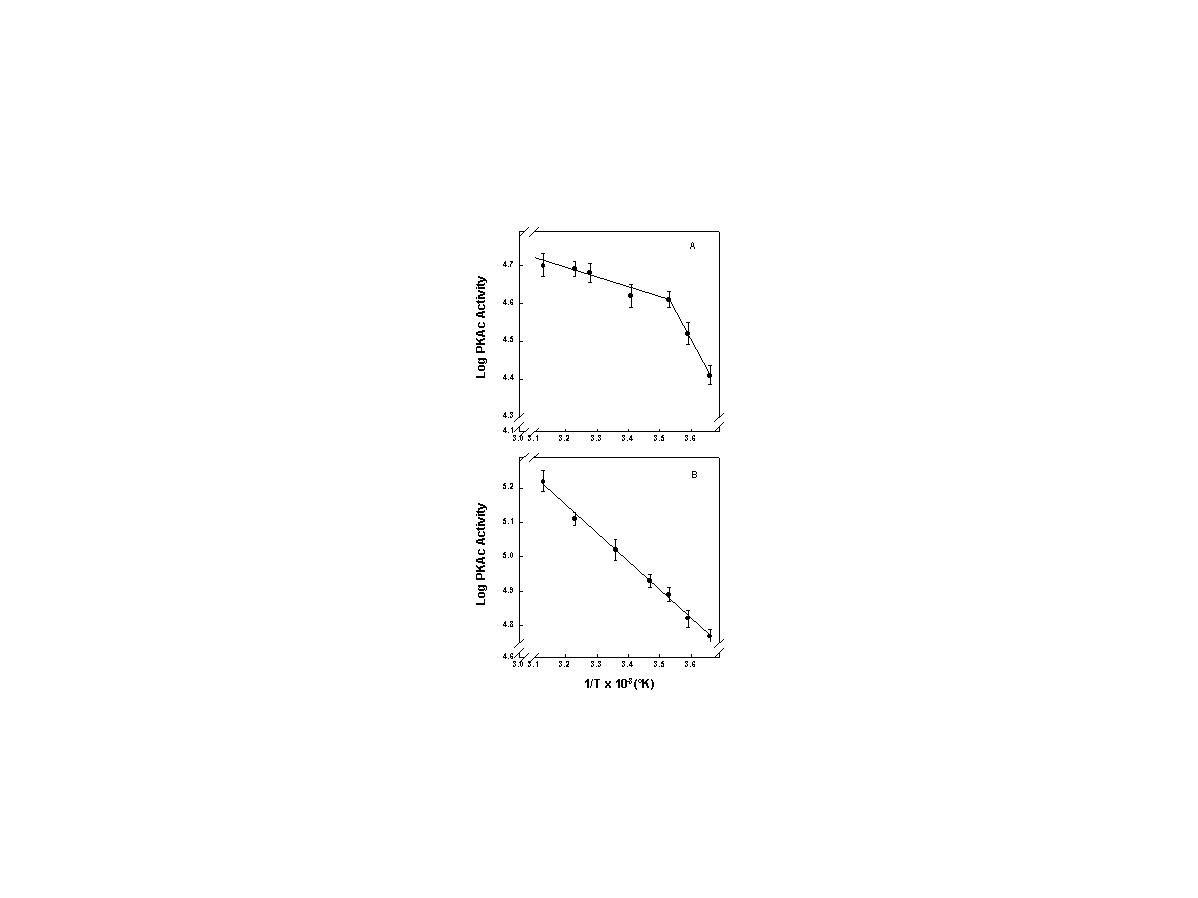 click to enlarge
Fig. 1: Arrhenius plots for bat skeletal muscle and porcine heart PKAc.
click to enlarge
Fig. 1: Arrhenius plots for bat skeletal muscle and porcine heart PKAc.
FIG. 1. Arrhenius plots for (A) purified bat skeletal muscle PKAc and (B) purified porcine heart PKAc. Maximal activities were measured at 5-10° C intervals over the range 0- 46°C. Bat PKAc showed a distinct break in the plot at 10°C. For the pig enzyme, the Arrhenius relationship was linear over the full temperature range tested with a calculated activation energy of Ea of 15.9 ± 0.3 kJ/mol (n=3). By contrast, bat PKAc showed a distinct break in the relationship at 10°C. The Ea for the reaction above 10°C was 5.6 ± 0.3 kJ/mol but below 10°C was 5-fold higher at 29.5 ± 1.7 kJ/mol (n=3). Data are means ± SEM, n = 3 separate enzyme preparations.
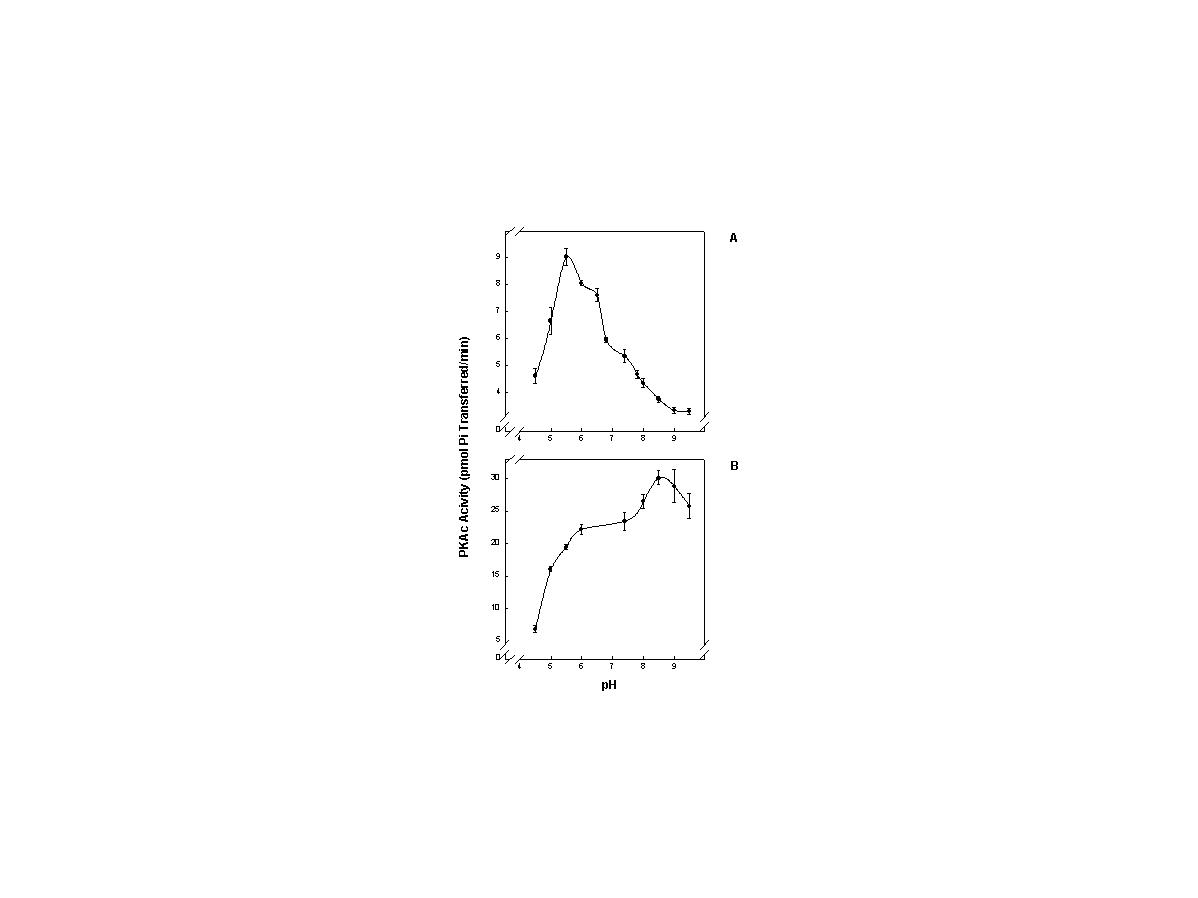 click to enlarge
Fig. 2: Effect of temperature on pH for purified PKAc from Myotis lucifugus skeletal muscle
click to enlarge
Fig. 2: Effect of temperature on pH for purified PKAc from Myotis lucifugus skeletal muscle
FIG. 2. The effect of assay pH on the activity of purified bat PKAc at 5°C (A) and 37°C (B). Total Mg2+ and ATP concentrations were varied to maintain constant concentrations of Mg-ATP and free Mg2+ at each temperature and pH. For pH curves, the standard 60µl assay mixture was modified by the addition of a 10µl aliquot of a buffer mixture (5 mM KH2PO4 + 5 mM K2HPO4, 10 mM imidazole, and 10 mM Tris base) that was pre-adjusted to one of the desired pH values. This changed assay pH to the desired value and this was confirmed by pH measurement after each assay was completed. The pH optimum was 8.5 at 37°C but this dropped dramatically to pH 5.5 when the assay temperature was decreased to 5°C. Data are means ± SEM for n = 3 separate enzyme preparations.
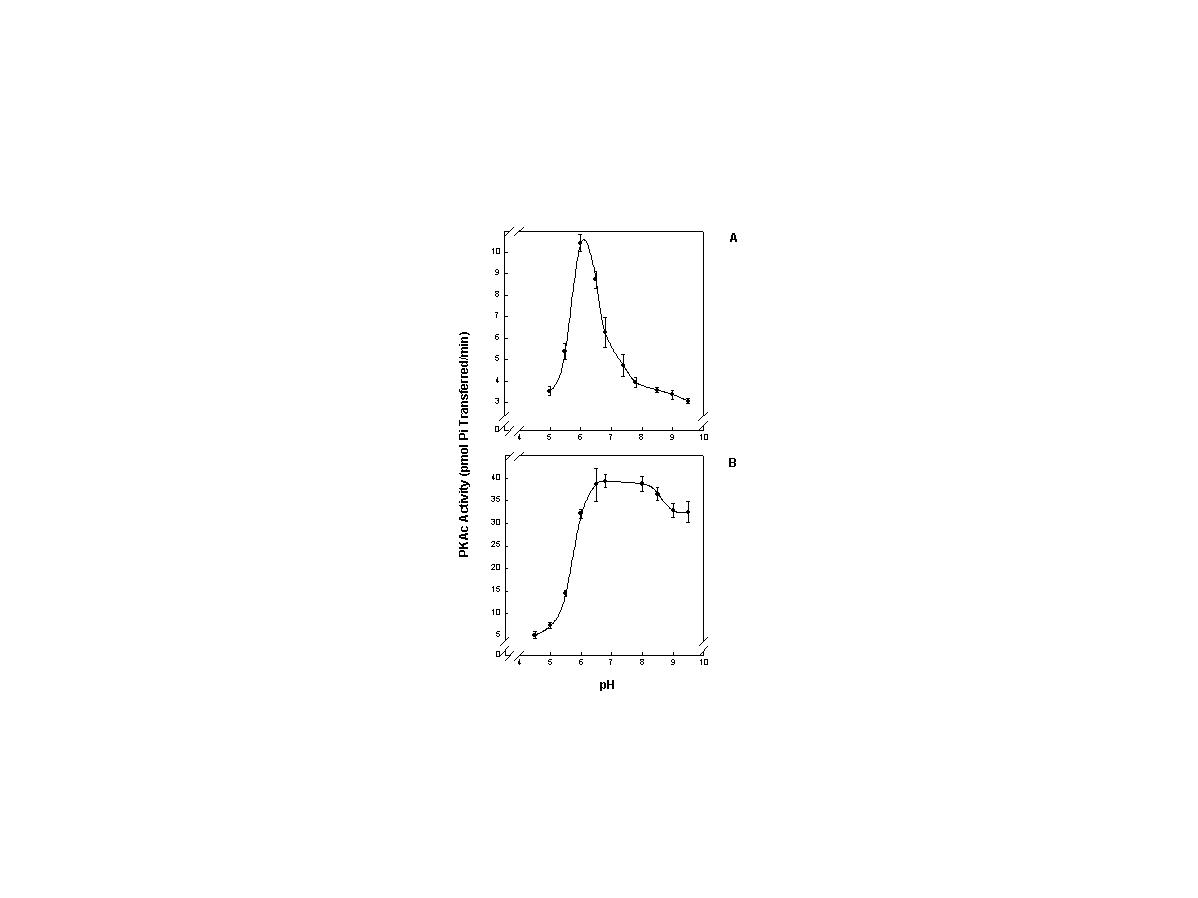 click to enlarge
Fig. 3: Effect of temperature on pH for purified PKAc from porcine cardiac muscle
click to enlarge
Fig. 3: Effect of temperature on pH for purified PKAc from porcine cardiac muscle
FIG. 3. The effect of pH on purified porcine heart PKAc activity at 5°C (A) and 37°C (B). Porcine PKAc showed a different response to temperature. A broad optimum from pH 6.5-8.0 occurred at 37°C whereas at 5°C a very narrow optimum was observed at pH 6.0. PKAc from both animals showed relatively broad pH optimal ranges at euthermic body temperature and quite narrow ranges at the lower temperature. Other information as in Fig. 3.
| <= Materials & Methods | RESULTS | Discussion & Conclussions => |
| Discussion Board | Next Page | Your Poster Session |