Cell Biology Poster Session
| INABIS '98 Home Page | Your Session | Symposia & Poster Sessions | Plenary Sessions | Exhibitors' Foyer | Personal Itinerary | New Search |
Results
Glucose utilization
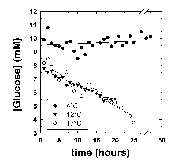
The effect of temperature on the rate of glucose disappearance is presented in Figure 1. Regression of the lines in Figure 1 showed that glucose was not utilized by R. sylvatica erythrocytes at 4°C (rate = -0.08 ± 0.04 mol/h/1015 cells, mean ± standard error from linear regression). At higher temperatures, glucose utilization increased to 0.91 ± 0.02 mol/h/1015 cells at 12°C and 1.27 ± 0.02 mol/h/1015 cells at 17°C. These values show a non-linear relationship between temperature and glucose utilization suggesting a profound blockage of glycolysis at 4°C. The extent of this blockage is illustrated by extrapolating the temperature curve between 12°C and 17°C to 4°C. The Q10 value of 2 over this temperature range predicts a glucose utilization rate of 0.52 mol/h/1015 cells at 4°C. The apparent blockage at 4°C could not be overcome by addition of 200 mM glucose to the NMR tube (Figure 2).
 Click here for enlarged Figure 1
Click here for enlarged Figure 1
Figure 1: Glucose disappearance measured at 4°C, 12°C and 17°C. The NMR tube contained 21.8, 19.2 and 17.8 mg/ml hemoglobin, respectively. All reactions were started by the addition of 13 ul of 10 mM [2-13C]D-glucose. The glucose concentration was measured by comparison of the relative glucose peak height at 76.7 ppm to the p-DP peak height at 127.5 ppm. This was calibrated with known concentrations of [2-13C]D-glucose measured under identical conditions. The relative rates of glucose disappearance were used to calculate absolute rates (see text).
 Click here for enlarged Figure 2
Click here for enlarged Figure 2
Figure 2: 13C-NMR spectra of R. sylvatica erythrocytes incubated at 4°C for 34h. Cells (approximately 20% haematocrit) were incubated with 200 mM [2-13C]D-glucose in a 5 mm NMR probe with a 2.5 mM capillary tube containing p-phenylenediamine (p-PD) used as a reference material for calculating concentrations of glucose and intermediates. Peaks at 106.6, 102.1 and 99.7 ppm were identified as the C-2 carbon of fructose by comparison with known standards run under the same conditions. Peaks at 75.0, 73.5 and 73.3 ppm were identified as glucose 6-phosphate (G6P) by comparison with the 13C-NMR spectra of unlabelled G6P (peaks were observed at 75.0, 73.4 and 73.3, among others). NMR peaks associated with F6P occurred at 66.8, 65.1 and 60.0 (unlabelled F6P showed peaks at 66.8, 65.1 and 60.0, among others). Abbreviations: [C-2]Fru, C-2 carbon of fructose; [C-2]Lactate, C-2 carbon of lactate; [C-2]Glc, C-2 carbon of glucose.
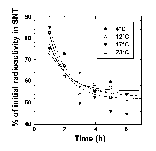
The location of the glucose-utilization blockage was investigated by two different methods: incubations with [U-14C]D-glucose and assignation of the NMR peaks to glycolytic intermediates. Incubations with [U-14C]D-glucose were used to test for a block at the glucose transporter. After incubation, the cells were isolated and the radioactivity of the supernatant (glucose not taken up) and pellet (glucose taken up) fractions was measured. Temperature had no effect on the rate or extent of glucose uptake over the first few hours (Figure 3). This shows that the rate of glucose transport was much faster than could be detected using the time course of Figure 3. It also demonstrates that glucose transport was not rate limiting under our conditions.
 Click here for enlarged Figure 3
Click here for enlarged Figure 3
Figure 3: Glucose uptake by R. sylvatica erythrocytes. Erythrocytes were incubated with 10 mM [14C-U]glucose at 4, 12, 17, and 23°C. Aliquots were removed and centrifuged and the radioactivity remaining in the supernatant (SNT) was plotted as a function of the experimental time course. Lines represent theoretical fits to an arbitrary exponential decay equation.
The NMR scans from the metabolism experiments were used to define the approximate location of the glycolytic blockage. Although only minor amounts of glycolytic intermediates accumulated during the metabolic time courses (approximately 5% of the initial glucose substrate), fructose (peaks at 106.6, 102.1 and 99.7 ppm) and glucose 6-phosphate (peaks at 75.0, 73.5 and 73.3 ppm) could be identified from the spectra (Figure 4). These results suggest that inhibition occurred at the PFK locus.
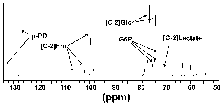 Click here for enlarged Figure 4
Click here for enlarged Figure 4
Figure 4: Figure 4 is identical to Figure 3 with the exception that the scale has been adjusted to maximize the 127.5 ppm peak of p-PD. This means that the full glucose peaks are not presented in this figure.
pH measurements
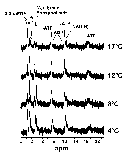
It is possible to calculate the pH of the cell milieu by monitoring the change in the internal free phosphate signal as a function of temperature and comparing this change to buffers of known pH at a given temperature (4). Following this procedure, R. sylvatica erythrocyte preparations were monitored at various temperatures using 31P-NMR spectroscopy and the peaks used to calculate the cellular pH at specific temperatures. Figure 5 shows the 31P-NMR spectra of the erythrocyte preparation at various temperatures. The peaks have been identified by comparison with literature values. As expected, the phosphate peak shifts as a function of temperature reflecting a change in the internal pH of the erythrocyte. Comparison of the peak height with standards, one can calculate erythrocyte pH values of 7.3 (4°C), 7.2 (8°C), 7.1 (12°C) and 7.0 (17°C). The overall change was approximately -0.025 units/°C, similar to that expected from the effect of temperature on the imidazole pKa (-0.017 units/°C).
 Click here for enlarged Figure 5
Click here for enlarged Figure 5
| <= Materials & Methods | RESULTS | Discussion & Conclussions => |
| Discussion Board | Next Page | Your Poster Session |