Invited Symposium: Neural Mechanism of Mammalian Vocalization
| INABIS '98 Home Page | Your Session | Symposia & Poster Sessions | Plenary Sessions | Exhibitors' Foyer | Personal Itinerary | New Search |
Introduction
There is widespread interest in the neural substrates of communication in nonhuman primates (NHP), due to their position as our closest relatives, and the overall similarities in brain structure between humans and NHP. However, nearly all work to date has used adult subjects. Because the developing brain is likely to be organized differently than the mature brain, and because the neural basis of the development of vocal communication in NHP is likely to provide important clues to the neural mechanisms of vocal communication in humans, we have begun a research program with this as the specific aim (e.g., ref. 1 ).
The present study is an extension of that work, and is focused on the alterations in vocal behavior during infancy in animals that had portions of inferior temporal cortex (area TE) removed during the neonatal period. We have reported that area TE ablations resulted in increased vocal behavior in infants examined when 1 to 1-1/2 years of age (1). Further, males with TE lesions produced more noisy calls than control males. Here, we report a comparison of the effects of the TE lesions on the vocal behavior in these animals when they were younger.
Materials and Methods
Subjects were infant Rhesus macaques, Macaca mulatta, aged 4 weeks to 16 months. All were individually housed in a colony room of the Laboratory of Neuropsychology, National Institute of Mental Health, on the main NIH campus in Bethesda, Maryland. Housing consisted of wire-mesh cages that permitted visual, auditory and limited somatosensory contact with other infants. During the first month, cotton towels were provided inside the cages, and the infants were handled several times daily by caretaker staff. Additionally, each infant received peer contact for up to 6 hr/day. Seven infants (5 males, 2 females) received bilateral ablations to area TE of the inferior temporal cortex. Seven others (4 males, 3 females) served as unoperated controls. Details regarding husbandry, surgical procedures and post-operative care can be found in ref. 2. A total of 66 recording sessions provided vocal data for analysis. Sessions typically lasted 5-10 minutes. Recording was performed sporadically, at times when the animals were not involved in other studies. Not all ages were represented for every individual, but the age range for both groups of subjects was similar. Recording sessions were performed in the morning, prior to feeding. Sessions involved transporting a subject from its home cage to another room inside a wire mesh transfer cage, placing it inside a reduced version of the Wisconsin General Testing Apparatus (approximately 1.0 x 0.5 x 0.5 m) and leaving it alone. No other monkeys or humans were visible or within hearing range during the recording session. Following the recording session, the subject was returned to its home cage. All vocalizations were recorded through a microphone placed at the open end of the WGTA onto a Marantz PMD 430 casette tape recorder (Marantz America, Roselle, Illinois). Vocalizations were scored by playing the tape into a Kay 5500 DSP Workstation (Kay Elemetrics Corp., Lincoln Park, New Jersey), and entering each call as ‘coo,’ ‘noisy call’ (leap, scream, noisy coo), or ‘other’ (everything else), following the call categorization scheme published earlier (3). Call type frequency distributions between the TE and C groups were tested for statistical significance using a nonparametric version of the Chi-squared test and Statview 4.5 software (Abacus Concepts, Berkeley, California).
Results
It was reported earlier (1) that TE animals around 1 year of age call at a higher rate than C animals of the same age. For the present study, the original data were reanalyzed, and some additional animals added. These additional data permitted examining males and females separately. Figure 1 compares the call rates of TE and C monkeys at an age range of 10-16 months, the oldest age range examined.
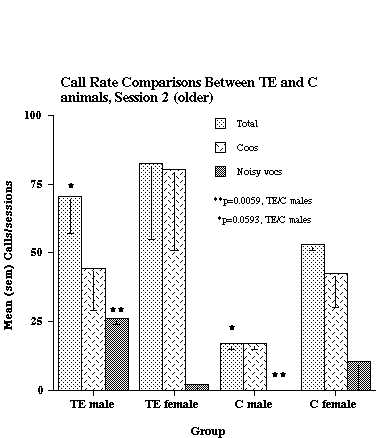
Figure 1. Call rate comparisons between TE and C animals at the oldest age range tested (10-16 months of age). The data shown are based on analyses from 2 TE males, 2 TE females, 2 C males, and 2 C females.
As shown in the figure, TE males called at a significantly higher rate than C males (Total calls and Noisy calls), but there was no significant difference in calling rate between TE and C females. Six subjects had data collected at an earlier age (6-9 months), and their results, along with that from 2 additional C animals, are shown in Figure 2.
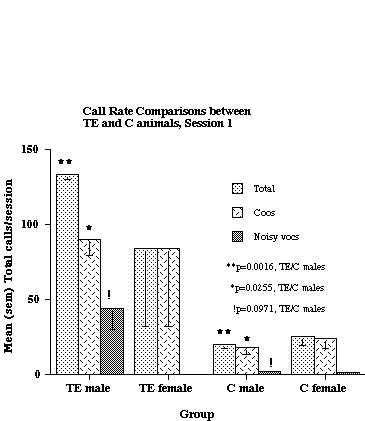
Figure 2. Call rate comparisons between TE and C animals when the subjects were 6-9 months of age. The data are based on analyses of 2 TE males, 2 TE females, 2 C males and 2 C females. Six of the 8 subjects were the same as were used for the analysis shown in Fig. 1.
As was the case at the older age, TE males called at a higher rate than C males, but there was no difference between TE and C females. With respect to the males only, there were significant differences in both Total Calls and Coos. Although there also was a trend for TE males to produce more Noisy calls than C males, this difference did not achieve statistical significance. Similar analyses of TE and C animals at still younger ages (4-8 weeks) did not reveal any significant differences between groups for either sex (data not shown).
Discussion and Conclusion
This study examined the effects of bilateral ablations of inferior temporal cortex (area TE) performed during the neonatal period on subsequent vocal behavior in the context of social separation. The differences between TE and control males at the oldest infant ages examined that we reported earlier (1) were largely confirmed. In addition, we found that similar differences existed, again only between TE and control males, at a younger age (6-9 months). Analyses of still younger animals (4-8 weeks of age) failed to demonstrate any differences in calling behavior between TE and control animals of either sex. These findings suggest that a developmental process is at work, presumably related to the functional role played by area TE and brain structures with which it is connected at different developmental stages. It is intriguing that other evaluations of these same animals have revealed a deficit in visual discrimination learning in females, but not in males, suggesting that area TE is more fully functional in females than in males at 3 months of age (2). Visual discrimination learning is impaired after TE lesions in adults of both sexes. Since the present study demonstrated a greater deficit in males following TE lesions, it is possible that area TE subserves other functions besides visual discrimination, at least during development, particularly in view of the evidence that lesions of area TE in infant macaques alter cortico-amygdala projections (4). Precisely why area TE lesions result in altered vocal behavior is unknown. It is possible that removal of this area compromises the ability of an infant to recognize the details of its environment, so that it becomes more reactive to perceived changes in a novel setting (such as occured when the animals were transported from their home cage to the recording room in the present study). However, in the above-cited study, it was female infants that showed the greater deficit in making visual discriminations. Since the amygdala has been implicated in a range of emotional behavior, it is possible that removing a major cortical input alters amygdalar circuitry in such a way as to affect emotional reactivity to mildly stressful challenges. Again, why males should be affected and not females is unclear. Neonatal androgen implants in infant females have been shown to slow the learning of visual tasks to a level typical of male infants (5). It would be valuable to test the effects of this procedure on the development of vocal behavior.
References
- Newman, JD and Bachevalier, J (1997). Neonatal ablations of the amygdala and inferior temporal cortex alter the vocal response to social separation in rhesus macaques. Brain Research, 758: 180-186.
- Bachevalier, J, Brickson, M, Hagger, C, and Mishkin, M. (1990). Age and sex differences in the effects of selective temporal lobe lesions on the formation of visual discrimination habits in rhesus monkeys (Macaca mulatta). Behav. Neurosci., 104: 885-899.
- Newman, JD (1995). Vocal ontogeny in macaques and marmosets: convergent and divergent lines of development. In: E. Zimmermann, J.D. Newman and U. Jürgens (editors), ‘Current Topics in Primate Vocal Communication,’ pp. 73-97, Plenum Press, New York.
- Webster, M, Ungerleider, LG, and Bachevalier, J. (1991). Lesions of inferior temoral area TE in infant monkeys alter cortico-amygdalar projections. NeuroReport, 2: 769-772.
- Hagger, C, and Bachevalier, J. (1991). Visual habit formation in 3-month-old monkeys (Macaca mulatta): reversal of sex difference following neonatal manipulations of androgens. Behavioural Brain Research, 45: 57-63.
| Discussion Board | Previous Page | Your Symposium |