Invited Symposium
| INABIS '98 Home Page | Your Session | Symposia & Poster Sessions | Plenary Sessions | Exhibitors' Foyer | Personal Itinerary | New Search |
Introduction
Melatonin Receptors In Reproductive Tissues: Evidence For The Multiple Sites Of Melatonin Action
The avian or mammalian pineal gland is the major source of melatonin in the circulation (Pelham et al., 1972; Pang and Ralph, 1975; Neuwelt and Lewy, 1983; see Pang et al., 1993b for review). In response to the diurnal light/dark cycle in the environment, the pineal gland synthesizes and secretes melatonin in a diurnal pattern with high levels in the dark period. As melatonin is a lipid soluble molecule, it has no barrier and reaches every part of the body. Thus, the daily cycle of melatonin in the blood readily conveys the environmental photoperiodic information to every tissue and organ and plays an important role in the synchronization of the daily and circannual rhythms in the body (see Reiter, 1993; Tang et al., 1996; Arendt, 1998 for review).
The Hypothesis - Multiple Sites of Melatonin Action on Reproduction:
Studies in the last forty years have established unequivocally that the seasonal reproductive cycle in animals is under melatonin regulation. However, the exact sites of melatonin action in the reproductive system are not clear. Theoretically, site(s) of melatonin action on the reproductive system can be the hypothalamus, pituitary, gonads, male and female reproductive tract, male accessory sex organs, mammary gland or any combination of the above targets (Pang et al., 1998). This allows the proposal of a hypothesis: Multiple sites of melatonin action on the reproductive system.
Recent investigations on melatonin receptors in the reproductive system have provided evidence in support of the above hypothesis. In this article, we would like to review the relevant findings related to the multiple-site hypothesis. It is hoped that this paper may facilitate discussion and further research in this area.
Evidence
Evidence For The Multiple Sites of Melatonin Action on Reproduction
The Hypothalamic-pituitary axis:
The hypothalamic-pituitary axis is the classical target of melatonin action. Fraschini and co-workers (1968) provide the first evidence for the involvement of hypothalamus on the antigonadal action of melatonin implants in the rat (Fraschini et al., 1968).
Subsequently, numerous studies have extended and provided support to the earlier data. A few of these findings are: changes in LH and FSH secretion following melatonin treatment (Kamberi et al., 1970; Bittman and Karsch, 1984) and modulation of storage and secretion of gonadotropin-releasing hormone (GnRH) from the hypothalamus by melatonin in vitro or in vivo (Kao and Weisz, 1977; Petterborg and Paull, 1984; Glass and Knotts, 1987). Using the specific melatonin radioligand, 2[125I]iodomelatonin, melatonin receptors have been localized in the suprachiasmatic nuclei (SCN) of many animals studied (Reppert et al., 1988; 1994; see Masson - Pevet et al., 1996a; 1996b for review).
In situ hybridization investigations demonstrated mRNA of Mel1a (mt1) melatonin receptor subtype in the SCN of animals and humans (Weaver and Reppert, 1996; Liu et al., 1997; Neu and Niles, 1997; Gauer et al., 1998;) and retinoid Z receptor beta mRNA in the rat SCN (Park et al., 1996). Modulation of SCN functions in vitro (Jiang et al., 1995; Starkey et al., 1995) or in vivo (Miguez et al., 1996; Masson-Pevet et al., 1996; Neu and Niles, 1997) changed the characteristics of SCN melatonin receptors. This is, in part, in accord with the studies in the Syrian hamster in which the photoperiodic response depends upon a melatonin driven rhythm of sensitivity to melatonin (Pitrosky and Pevet, 1987).
The above findings confirm the physiological functions of SCN melatonin receptors and the importance of hypothalamus in melatonin action. However, the nature of SCN involvement in the reproductive action of melatonin remains unknown. In addition to the SCN, 2[125I]iodomelatonin binding has been reported in other brain sites such as the paraventricular nuclei (Recio et al., 1996; Pevet et al., 1996; Masson-Pevet et al., 1996), ventromedial hypothalamic area and the premammillary hypothalamic area emcompassing the premammillary and tuberomammillary nuclei (Chabot et la., 1998; Malpaux et al., 1998). Are these sites important for the melatonin reproductive action? It was suggested that colocalization of melatonin receptors and GnRH in hypothalamic neurons may provide important insights on the neural substrates involved in the melatonin action on reproduction (Pang et al., 1998).
Alternatively, the melatonin influences on GnRH neurons may be indirect and the population of dopaminergic neurons with axons projecting to the median eminence has been proposed as one of candidate interneurons involved (Malpaux et al., 1997).
Martin and Klein reported the first direct melatonin action on the pituitary. Melatonin inhibited the GnRH-induced LH release by neonatal rat anterior pituitary cells in vitro (Martin and Klein, 1976) or hamster pituitary in vivo (Wun et al., 1986). In neonatal rats, the pituitary has a high density of melatonin receptors (Vanecek, 1988). Melatonin decreased cAMP and cGMP accumulation in the rat pituitary cells (Vanecek and Vollrath, 1989). In addition, melatonin also inhibited GnRH-induced Ca++ mobilization and Ca++ influx in the gonadotrophs via the high-affinity membrane-bound melatonin receptors (Slanar et al., 1987). These melatonin receptors in neonatal rat pituitary, however, cannot account for the regulation of seasonal reproduction in the mature rodents. In adult animals, high densities of 2[125I]iodomelatonin binding sites or melatonin receptors have only been detected in the pars tuberalis (PT) of the pituitary stalk with no significant binding in other pituitary loci (Vanecek et al., 1987; Morgan, 1989; Masson-Pevet et al., 1996a). In rodents, there is a seasonal change in PT melatonin receptors with significantly lower densities in the short photoperiod or melatonin-treated animals. It was suggested that PT melatonin receptors in rodents may be important in the control of seasonal reproduction in photoperiodic species (Masson-Pevet et al., 1996b). However, the PT is not part of the anterior pituitary where the gonadotrophs s are located. In addition, PT cells are different from other pituitary cells ultrastructurally (Gross, 1984; Morgan and Williams, 1996). How the PT regulates the reproductive system remains an enigma (Masson-Pevet et al., 1996b). It was suggested that ovine pars tuberalis cells secrete a factor ('tuberalin') that exerts hormonal control over prolactin synthesis and release from the pars distalis lactotrophs. Conversely, in the sheep, melatonin has been reported to modulate the GnRH-induced increase in LH output from the ovine PT but not the pars distalis in vitro (Skinner and Robinson, 1997). There may be species di fference in the structure of PT in the pituitary. Apparently, more studies have to be conducted before the question of how the PT melatonin receptors affect seasonal reproduction can be fully addressed (Pang et al., 1998).
In short, the importance of hypothalamic-pituitary axis on the reproductive function of melatonin is well established. However, further research is warranted to clarify the hypothalamic or pituitary sites of melatonin action as well as the signaling mechanism of the hormone responsible for the regulation of seasonal reproduction.
Testis and Ovary:Melatonin alters the morphology, steroidogenesis or cGMP production of testicular tissues, Leydig cells (Ellis, 1972; Ng and Lo, 1988; Persengiev and Kehajova, 1991; Niedziela et al., 1995; Valenti et al., 1997) corpus luteum and granulosa cells in vitro (MacPhee et al., 1975; Fiske et al., 1984; Baratta and Tamanini, 1992; Murayama et al., 1997). The above findings indicate a direct melatonin action on the testis and ovary. 2[125I]Iodomelatonin or [3H]melatonin binding sites have been identified in the testes and ovaries of birds (Ayre et al., 1992; 1994; Wang et al., 1992; Ayre and Pang, 1994; Murayama et al., 1997), Leydig cells of rats (Valenti et al., 1997) or granulosa cells from human preovulatory follicles (Yie et al., 1995). These picomolar affinity, G-protein-coupled and specific gonadal 2[125I]iodomelatonin binding sites satisfy the pharmacokinetic properties of specific receptors (Ayre et al., 1992; 1994; Wang et al., 1992; Ayre and Pang, 1994; Valenti et al., 1997; Yie et al., 1995; Valenti et al., 1997). Different affinities have been reported in different laboratories (Ayre et al., 1992; 1994; Wang et al., 1992; Ayre and Pang, 1994; Murayama et al., 1997). This may be the result of the differences in the time of tissue sampling, species of animal used and the methods employed. Autoradiography (ARG) indicated that these 2[125I]iodomelatonin binding sites were widely distributed throughout the bird testes but highly localized in the bird ovarian follicles (Ayre et al., 1994; Ayre and Pang, 1994). Short photoperiod led to a down regulation of 2[125I]iodomelatonin binding in the quail testes (Pang et al., 1993). Preliminary studies in our laboratory indicated that 2[125I]iodomelatonin binding in the rodent testis was low or non-detectable (Ayre, 1993). Using purified Leydig cells of rats, Valenti and coworkers (1997) reported melatonin receptors with a maximum number of binding sites (Bmax) of 46.7 fm ol/mg protein and an equilibrium dissociation constant (Kd) of 88.7 pM. These melatonin receptors are coupled to a pertussis toxin-sensitive G-protein and are responsible for the inhibition of forskolin and LH induced testosterone secretion by the rat Leydig cells in vitro (Valenti et al., 1997). It is highly possible that melatonin receptors in mammalian testis are expressed at a lower level than that in birds. Do these gonadal melatonin receptors mediate the antigonadal action of melatonin on rodent reproduction and/or the progonadal action of melatonin on sheep breeding? What are the mechanisms involved?Male Reproductive Tract - Epididymis:
The epididymis is part of the male reproductive tract. It creates a microenvironment for the transport, maturation and storage of spermatozoa. Through some unknown processes, the sperm acquires its ovum fertilization ability during its passage via the epididymis. High affinity epididymal melatonin receptors are localized in the rat corpus epididym is (Yu et al., 1994; Williams and Hannah, 1995; Shiu et al., 1996a; 1996b). In situ hybridization has demonstrated mRNAs of Mel1a and Mel1b (MT2) melatonin receptors in epithelial cells of rat corpus epididymis (Shiu et al., 1997; Li et al., 1998). Testosterone reversed the castration-decreased epididymal 2[125I]iodomelatonin binding density in rats (Shiu et al., 1996b; 1997). Under an in vitro condition, melatonin reduced forskolin increased cAMP accumulation by rat epididymal cells (Li et al., 1998). These findings propose a direct melatonin action, via the activation of specific Gi protein-coupled receptors, on the regulation of corpus epididymal physiology and reproductive functions in rats (Shiu et al., 1997). What is the effect of pinealectomy on reproduction? Does epididymal melatonin receptor affect the process of sperm maturation in the epididymis? What is the mechanism of action? Male Reproductive Tract - Vas Deferens:
The vas deferens serve s as a conduit for the the transport of epididymal sperm to the urethra. During copulation, the vas deferens together with epididymis, ampulla and urethra contract and cause semen ejaculation into the vagina. Acetylcholine is involved in the vas deferens contraction. There is a diurnal rhythm on the acetylcholine-induced prostatic vas deferens contraction that is also potentiated by melatonin (Carneiro et al., 1991). Furthermore, melatonin also increased the calcium-dependent release of [3H]-norepinephrine in the rat vas deferens (Carneiro et al., 1993a). The above processes may be melatonin receptors mediated as ARG studies have demonstrated 2[125I]iodomelatonin binding in the mucosa (epithelial cells and lamina propria) and internal longitudinal muscle layer of the prostatic but not the epididymal portion of rat vas deferens (Carneiro et al., 1993b). However, collaborative data on the molecular, pharmacological, and physiological characteristics of melatonin receptors in the vas defere ns would be important. Accessory Sex Organs - Prostate Gland:
Prostates, seminal vesicles, ampullary glands, and coagulating glands are the accessory sex organs in rodents. The removal of prostates significantly reduced fertility in rats, golden hamsters and mice (Pang et al., 1979; Queen et al., 1981; Chow et al., 1986; Peitz and Olds-Clarke, 1986). Ablation of prostates or all accessory sex organs, however, did not change the fertilizing ability of spermatozoa (Chow and Pang, 1989) nor the mating behavior in Golden Hamsters (Chow et al., 1989). The major cause of fertility impairment in prostate-removed hamsters is the failure of normal embryonic development (O et al., 1988). Similar to rodents, the prostates together with seminal vesicles and bulbourethral glands constitute the accessory sex organs in humans. Whether the human prostates are essential for effective male reproduction has not been examined. However, in light of the findings in rodents, the importance of prostates on human fertility cannot be underestima ted.
Melatonin treatment induced regression of the prostate and atrophy of secretary cell organelles in the accessory sex organs including the prostates (Chow and Pang., 1989). The inhibitory effect of melatonin on the prostate may be mediated through other endocrine systems. Alternatively, a direct action of melatonin has also been proposed (Chow and Pang, 1989). Recent evidence has suggested that the prostate gland may be another site of melatonin action. High-affinity, specific and G protein-coupled melatonin receptors have been reported in the cytosol of human prostate glandular epithelial cells. These receptors may be responsible for the significant inhibitory effect of melatonin on human prostatic cell growth in vitro (Gilad et al., 1996). Melatonin receptors in human benign prostate epithelial cells augmented cAMP and inhibited cGMP through pertussis toxin-sensitive and cholera toxin-sensitive G-proteins respectively. In addition, the melatonin-induced decrease in cGMP may result in a decrease in DNA synthesis in the prostate epithelial cells (Gilad et al., 1998a). Dihydrotestosterone and estradiol reduced the ability of melatonin to suppress benign prostatic cell growth and viability (Gilaad et al., 1997). Specific binding of 2[125I]iodomelatonin has also been demonstrated in the microsomal fraction of rat ventral prostate cells. These binding sites may mediate the suppressive effect of melatonin on testosterone-dependent prostate growth in rats (Gilad et al., 1998b). What is the relationship between prostate tumor and pineal melatonin in aged animals?
Female Reproductive Tract - Uterus:ARG studies demonstrated 2[125I]iodomelatonin binding in the endometrial stroma of uterus. However, it is not possible to identify if 2[125I]iodomelatonin binding is present in the glandular epithelium, vascular endothelium, stromal cells or other cells in the stroma of uterus (Zhao et al., 1998a). The binding has a Kd of 14.6 pM and a density of 0.45 fmol/mg protein. The number of uterine melatonin binding sites varied during the estrous cycle (Zhao et al., 1998b). It was suggested that melatonin may regulate the endometrial vascular permeability and decidualization (Zhao et al., 1998a). Extensive studies on the uterine putative melatonin receptors should be further conducted.Mammary Gland:
2[125I]Iodomelatonin binding has been demonstrated on murine mammary gland membranes and mammary tumour cells (Blask et al., 1992; Recio et al., 1994a; 1994b). The binding affinity (Kd) was 1.28-3.05 nM and the density (Bmax) was 31-311 fmol/mg protein for the murine mammary membranes (Recio et al., 1994a). The binding displayed typical receptor characteristics with changes in the binding parameters at different times of the day, stages of postnatal period, estrous cycle, pregnancy and lactation (Recio et al., 1994b). Preliminary ARG studies suggest that 2[125I]Iodomelatonin binding is located on the epithelial structures of the ductal tree. Sub-nanomolar concentrations of melatonin modulate the growth, increase cGMP and decrease cAMP synthesis in murine mammary glands in vitro (Sanchez-Barcelo et al., 1991; Cardinali et al., 1992; Recio et al., 1994b). However, in comparison with the affinity of 6-hydroxymelatonin, the melatonin metabolite, the affinity of melatonin was relatively low to these receptors. The possibility that 6-hydroxymelatonin is the active hormone on the mammary tissues has to be considered.
Advantages
Possible Advantages of Multiple Sites of Melatonin Action
Depending on the species studied, melatonin-induced biological responses and/or the presence of melatonin receptor subtypes have been demonstrated in a combination of reproductive tissues including the testis, Leydig cells, ovary, granulosa cells, epididymis, vas deferens, prostate, and mammary gland. In some tissues such as the epididymis, mRNA of both Mel1a and Mel1b melatonin receptor subtypes have been demonstrated in the same tissue. Whether they are expressed in the same cell remains to be investigated. In most tissues studied, densities of tissue melatonin receptors change in response to external or internal stimulation. In addition, the changes in binding properties with alterations in physiological conditions further support that these tissue 2[125I]iodomelatonin binding sites are melatonin receptors with physiological functions.
The demonstration of 2[125I]iodomelatonin binding sites or melatonin receptors in more than one reproductive tissue in the rat and other species supports the hypothesis of multiple sites of melatonin action on the reproductive system. Melatonin action on multiple reproductive sites may generate a cooperative, synergistic or summative effect on the reproductive system constituting a robust combination of photoperiodic control on animal reproduction. This maximizes the use of a reliable environmental information (the photoperiod) on reproduction by the regulation of many components of the reproductive system. This robust controlling mechanism will furnish the successful birth and upbringing of young during the optimal season and ensures the survival of species (reproduction) (Pang et al., 1998).
Final Remarks
Theoretical considerations have allowed the hypothesis of 'Multiple sites of melatonin action on the reproductive system'. In line with the above proposal, accumulated data have suggested that direct melatonin action may occur in more than one reproductive tissue, from the level of hypothalamus to the levels of reproductive tracts and mammary gland (Figure 1). The affinities of many melatonin receptors reported are in the picomolar range. Given the low Kd values (10-30 pmol/L) reported for some melatonin receptors and the circulating level of melatonin in the day time ranged from 10-50 pg/ml (about 40-200 pmol/L) for most animals studied (Pang et al., 1993), it has been argued that 'melatonin is always acting on the target tissues irrespective of day-night rhythms of its secretion.
If endogenous melatonin rhythms are concerned in its action on target tissues, the extremely high affinity receptor may not be of physiological significance' (Murayama et al., 1997). In our opinion, two points should be noted: 1). The Kd values of melatonin receptors are obtained by 2[125I]iodomelatonin binding, not melatonin binding and 2-iodomelatonin has a much higher affinity than melatonin (Pang, CS et al., 1993; Yong et al., 1993; Poon et al., 1994); and 2). When the effects of cations are studied, physiological concentrations of cations cause a lowering of the melatonin receptor affinity (Pang, CS et al., 1996; Wan et al., 1997). Under physiological condition, melatonin receptors are soaked in the body fluid with high cncentrations of cations and metabolites, a higher Kd value can be predicted. The above points and others such as assay temperature have to be considered when the significance of endogenous melatonin level and melatonin receptor affinity is evaluated. In addition, with the exception of the hypothalamic-pituitary axis, other findings are mostly in their initial stages and demonstrated by one or two laboratories. Collaborative data from more laboratories would be important.
Earlier data only provide the preliminary information favoring the hypothesis of multiple sites of melatonin action. Many questions remain to be answered. Some of these questions are: What are the molecular, biochemical, and/or pharmacological characteristics of these melatonin receptors? What are the physiological roles of these melatonin receptors on reproduction? What are the signal transduction mechanisms invloved? How are these melatonin receptors regulated? Do they cross-talk with other chemical messagers? Some of the above questions have been addressed while others remain to be investigated. Answers to the above and other questions may provide additional information necessary for the acceptance or rejection of the Multiple-site hypothesis proposed.
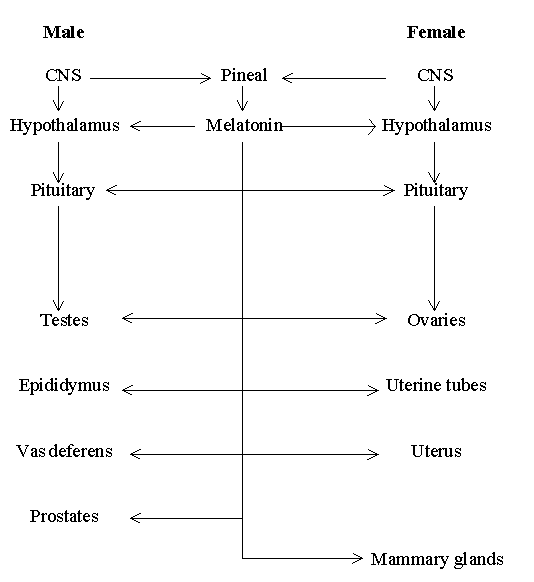
Figure 1. Multiple sites of melatonin action on the reproductive system, from the level of hypothalamus to the levels of reproductive tracts and mammary gland (modified from Pang et al., 1998)
References
Arendt, J (1998) Melatonin and the pineal gland: influence on mammalian seasonal and circadian physiology. Rev Reprod, 3(1):13-22
Ayre, EA (1993)The characterization, localization and physiological regulation of [125I]iodomelatonin binding sites in the gonads. The University of Hong Kong Thesis, Hong Kong.
Ayre, EA Yuan, H Pang, SF (1992) The identification of [125I]iodomelatonin binding sites in the testes and ovaries of the chicken (Gallus domesticus). J Endocrinol, 133:5-11.
Ayre, EA Pang, SF (1994) 2-[125I]iodomelatonin binding sites in the testis and ovary: Putative melatonin receptors in the gonads. Biol Signals, 3:71-84.
Ayre, EA Wang, ZP Brown, GM Pang, SF (1994) Localization and characterization of [125I]iodomelatonin binding sites in duck gonads. J Pineal Res, 17: 39-47.
Baratta, M Tamanini, C (1992) Effect of melatonin on the in vitro secretion of progesterone and estradiol 17b by ovine granulosa cells. Acta Endocrinol, 127:366-370.
Bittman, EL Karsch, FJ (1984) Nightly duration of pineal melatonin secretion determines the reproductive response to inhibitory day length in the ewe. Biol Reprod, 30:585-593.
Blask, DE Lemus-Wilson, AM Wilson, ST (1992) Breast cancer: a model system for studying the neuroendocrine role of pineal melatonin in oncology. Biochem Soc Trans, 20:309-311.
Cardinali, DP Bonanni Rey, RA Mediavilla, MD Sanchez-Barcelo, EJ (1992) Diurnal changes in cyclic nucleotide response to indoles in murine mammary glands. J Pineal Res, 13:111-116.
Carneiro, RCG Cipolla-Neto J Markus, RP (1991) Diurnal variation of the rat vas deferens contraction induced by stimulation of presynaptic nicotinic receptors and pineal function. J Pharmacol Exp Ther, 259:614-619.
Carneiro, RCG Markus, RP Dubocovich, ML (1993a) Presynaptic modulation by melatonin of the nicotine-induced calcium-dependent release of norepinephrine from rat vas deferens. Biol Signals, 2: 199-206.
Carneiro, RCG Markus, RP Dubocovich, ML (1993b) 2-[125I]iodomelatonin binding sites in the rat vas deferens. Biol Signals, 2:194-198.
Chabot, V Caldani, M de Reviers, MM Pelletier, J (1998) Localization and quantification of melatonin receptors in the diencephalon and posterior telencephalon of the sheep brain. J Pineal Res, 24(1):50-7
Chow, PH Pang, SF (1989) The Male accessory sex glands, fertility and melatonin. Adv Pineal Res, 3:221-224.
Chow, PA Pang, SF Ng, KW Wong, TM (1986) Fertility, fecundity, sex ratio and the accessory sex glands in the Golden Hamster. Int J Andrology, 9:312-320
Ellis, LC (1972) Inhibition of rat testicular androgen synthesis in vitro by melatonin and serotonin. Endocrinology, 90:17-28.
Fiske, VM Parker, KL Ulmer, A Seng, HO Aziz, N (1984) Effect of melatonin alone or in combination with human chorionic gonadotropin or ovine luteinizing hormone on the in vitro secretion of estrogen or progesterone by granulosa cells of rats. Endocrinology, 114:407-410.
Fraschini, F Mess, B and Martini, L (1968) Pineal gland, melatonin and the control of luteinizing hormone secretion. Endocrinology, 82:919-924.
Gauer, F Schuster, C Poirel, VJ Pevet, P Masson-Pevet, M (1998) Cloning experiments and developmental expression of both melatonin receptor Mel1A mRNA and melatonin binding sites in the syrian hamster suprachiasmatic nuclei. Mol Brain Res, 60(2):193-202
Gilad, E Laudon, M Matzkin, H Pick, E Sofer, M Braf, Z Zisapel, N (1996) Functional melatonin receptors in human prostate epithelial cells. Endocrinology, 137:1412-1417.
Gilad, E Laudon, M Matzkin, H Zisapel, N (1998a) Evidence for a local action of melatonin on the rat prostate. J Urol, 159(3):1069-73
Gilad, E Matzkin, H Zisapel, N (1997) Interplay between sex steroids and melatonin in regulation of human benign prostate epithelial cell growth. Clin Endocrinol Metab, 82(8):2535-41
Gilad, E Pick, E Matzkin, H Zisapel, N (1998b) Melatonin receptors in benign prostate epithelial cells: evidence for the involvement of cholera and pertussis toxins-sensitive G proteins in their signal transduction pathways. Prostate, 35(1):27-34
Glass, DJ Knotts, LK (1987) A brain site for the antigonadal action of melatonin in the white-footed mouse (Peromyscus leucopus): Involvement of the immunoreactive GnRH neuronal system. Neuroendocrinology, 40:48-55.
Gross, DS (1984) The mammalian hypophysial pars tuberalis: A comparative immunocytochemical study. Gen. Comp Endocrinol, 56:283-298.
Jiang, ZG Nelson, CS Allen, CN (1995) Melatonin activates an outward current and inhibits Ih in rat suprachiasmatic nucleus neurons. Brain Res, 687:125-132.
Kamberi, IA Mical, RS Porter, JC (1970) Effect of anterior pituitary perfusion and intraventricular injection of catecholamines and indoleamines on LH release. Endocrinology, 87:1-12.
Kao, LWL Weisz, J (1977) Release of gonadotrophin-releasing hormone (GnRH) from isolated, perifused medial-basal hypothalamus by melatonin. Endocrinology, 100:1723-1726.
Laudon, M Gilad, E Matzkin, H Braf, Z Zisapel, N (1996) Putative melatonin receptors in benign human prostate tissue. J Clin Endocrinol Metab, 81:1336-1342.
Li, L Xu, JN Pang, SF Shiu SYW (1998) Melatonin modulates cAMP signaling in rat epididymal epithelial cells. FASEB J, 12(5):A686
Liu, C Weaver, DR Jin, X Shearman, LP Pieschl, RL Gribkoff, VK Reppert, SM (1997) Molecular dissection of two distinct actions of melatonin on the suprachiasmatic circadian clock. Neuron 19(1):91-102
MacPhee, AA Cole, FE Rice, BF (1975) The effect of melatonin on steroidogenesis by the human ovary in vitro. J Clin Endocrinol Metab, 40:688-696.
Malpaux, B Viguie, C Skinner, DC Thiery, JC Chemineau, P (1997) Control of the circannual rhythm of reproduction by melatonin in the ewe. Brain Res Bull, 44(4):431-8
Malpaux, B Daveau, A Maurice-Mandon, F Duarte, G Chemineau, P (1998) Evidence that melatonin acts in the premammillary hypothalamic area to control reproduction in the ewe: presence of binding sites and stimulation of luteinizing hormone secretion by in situ microimplant delivery. Endocrinology, 139(4):1508-16
Martin, J.E. and Klein, D.C. (1976) Melatonin inhibition of the neonatal pituitary response to luteinizing hormone-releasing factor. Science 191:301-302.
Masson-Pevet, M Bianchi, L Pevet, P (1996a) Circadian photic regulation of melatonin receptor density in rat suprachiasmatic nuclei: comparison with light induction of fos-related protein. J Neuroscience Res, 43:632-637.
Masson-Pevet, M Gauer, F Recio, J (1996b) Melatonin receptors, pars tuberalis and photoperiodic response. Front Horm Res, 21:84-89.
Miguez, JM Martin, FJ Lema, M Aldegunde, M (1996) Changes in serotonin level and turnover in discrete hypothalamic nuclei after pinealectomy and melatonin administration to rats. Neurochem International, 29:651-658.
Morgan PJ, Lawson W, Davidson G, Howell HE (1989) Guanine nucleotides regulate the affinity of melatonin receptors on the ovine pars tuberalis. Neuroendocrinology 50(3):359-62
Morgan, PJ Williams, LM (1996) The pars tuberalis of the pituitary: a gateway for neuroendocrine output. Rev Reprod, 1(3):153-61
Murayama, T Kawashima, M Takahashi, T Yasuoka, T Kuwayama, T Tanaka, K (1997) Direct action of melatonin on hen ovarian granulosa cells to lower responsiveness to luteinizing hormone. Proc Soc Exp Biol Med, 215(4):386-92
Neu, JM Niles, LP (1997) A marked diurnal rhythm of melatonin ML1A receptor mRNA expression in the suprachiasmatic nucleus. Brain Res Mol Brain Res. 49(1-2):303-6
Neuwelt EA, Lewy AJ (1983) Disappearance of plasma melatonin after removal of a neoplastic pineal gland. N Engl J Med 308(19):1132-5
Niedziela, M Lerchl, A Nieschlag, E (1995) Direct effects of the pineal hormone melatonin on testosterone synthesis of Leydig cells in Djungarian hamsters (Phodopus sungorus) in vitro. Neuroscience Lett 201:247-250
Ng, TB Lo, L (1988) Inhibitory actions on pineal indoles on steroidogenesis in isolated rat Leydig cells. J Pineal Res, 5:229-243.
O, WS Chen, HQ Chow, PH (1988) Effects of male accessory sex glands on early embryonic development in the golden hamster. J Reprod. Fertil, 56:129-132.
Pang, CS Brown, GM Tang, PL. Cheng, KM Pang, SF (1993) 2-[125I]iodomelatonin binding sites in the lung and heart: A link between the photoperiodic signal, melatonin, and the cardio-pulmonary system. Biol. Signals 2: 228-236
Pang, CS Tang, LP Song, Y Brown GM Pang SF (1996) 2-[125I]iodomelatonin binding sites in the quail heart: Characteristics, distribution and modulation by guanine nucleotides and cations. Life Sci. 58: 1047-1057
Pang, SF Chow, PH Wong, TM (1979) The role of seminal vesicles, coagulating glands and prostate glands on the fertility and fecundity of mice. J Reprod Fertil, 56:129-132.
Pang, SF Cheng, KM Pang, CS Wang, ZP Brown, GM (1993) Differential effects of short photoperiod on specific binding of [125I]iodomelatonin in the testis and brain of quail. Biol Signals, 2:146-154.
Pang, SF Lee, PPN Chan, YS Ayre, EA (1993b) Melatonin secretion and its rhythms in biological fluids. In Melatonin: Biosynthesis, Physiological Effects and Clinical Applications. (eds Yu, H.S. and Reiter, R.J.) pp. 129-153, CRC Press, Boca Raton.
Pang, SF Li, L Ayre, EA Pang CS Shiu SYW (1998) Neuroendocrinology of melatonin in reproduction. J Chem Neuroanatomy, 14:157-166
Pang, SF Ralph, CL (1975) Pineal and serum melatonin activities at midday and midnight following pinealectomy or castration in male rats. J. Exp. Zool, 194:275-280
Park, HT Baek, SY Kim, BS Kim, JB Kim, JJ (1966) Developmental expression of 'RZR beta, a putative nuclear-melatonin receptor' mRNA in the suprachiasmatic nucleus of the rat. Neurosci Lett, 217:17-20.
Peitz, B Olds-Clarke, P (1986) Effects of seminal vesicle removal on fertility and uterine sperm motility in the house mouse. Biol Reprod, 35:608-617.
Persengiev, S Kehajova, J (1991) Inhibitory action of melatonin and structurally related compounds on testosterone production by mouse Leydig cells in vitro. Cell Biochem Funct, 9:281-286.
Petterborg, LJ Paull, WK (1984) An immunocytochemical study of the luteinizing hormone-releasing hormone (LHRH) system in the white-footed mouse: Effect of blinding and melatonin. J Pineal Res 1, 371-380.
Pitrosky, B Pevet, P (1997) The photoperiodic response in Syrian Hamster depends upon a melatonin-driven rhythm of sensitivity to melatonin. Biol Signals, 6:264-271
Poon, AMS Liu, CM Pang, CS Brown, GM Pang, SF (1994) Evidence for a direct action of melatonin on the immune system. Biol Signals 3: 107-117
Queen, K Dhabuwala, CB Pierrepoint, CG (1981) The effects of the removal of the various accessory sex glands on the fertility of the male rats. J Reprod Fertil, 62:423-436.
Recio, J Cardinali, DP Sanchez-Barcelo, EJ (1994a) 2[125I]Iodomelatonin binding sites in murine mammary tissue. Biol Signals, 3:85-90.
Recio, J Mediavilla, MD Cardinali, DP Sanchez-Barcelo, EJ (1994b) Pharmacological profile and diurnal rhythmicity of 2-[125I]iodomelatonin binding sites in murine mammary tissue. J Pineal Res, 16:10-17.
Recio, J Pevet, P Masson-Pevet, M (1996) Serotonergic modulation of photically induced increase in melatonin receptor density and Fos immunoreactivity in hte suprachiasmatic nuclei of the rat. J Neuroendocrinol, 8(11):839-45.
Reiter, RJ (1993) The melatonin rhythm: Both a clock and a calendar. Experientia, 49:654-664.
Reppert SM, Weaver DR, Rivkees SA, Stopa, EG (1988) Putative melatonin receptors in a human biological clock. Science 242(4875):78-81
Reppert, SM Weaver, DR Ebisawa, T (1994) Cloning and characterization of a mammalian melatonin receptor that mediates reproductive and circadian responses. Neuron, 13:1177-1185.
Sanchez-Barcelo, EJ Mediavilla, MD Zinn, SA Buchanan, BA Chapin, LT Tucker, HA (1991) Melatonin suppression of mammary growth in heifers. Biol Reprod, 44:875-879.
Shiu, SYW Yu, ZH Chow, PH Pang, SF (1996a) Putative melatonin receptors in the male reproductive tissues. Front Horm Res, 21:90-100.
Shiu, SYW Chow, PH Yu, ZH Tang, F Pang, SF (1996b) Autoradiographic distribution and physiological regulation of 2-[125I]iodomelatonin binding in rat epididymis. Life Sci, 59:1165-1174.
Shiu, SYW Li, L Wong, JTY Pang, SF (1997) Biology of G protein-coupled melatonin receptors in the epididymis and prostate of mammals. Chinese Med J, 110:648-655
Shiu, SY Pang, SF (1998) An updated phylogenetic analysis of vertebrate melatonin receptor sequences: reflection on the melatonin receptor nomenclature by the nomenclature subcommittee of the international union of pharmacology. Biol Signals Recept, 7(4):244-8
Skinner DC, Robinson JE (1997) Luteinising hormone secretion from the perifused ovine pars tuberalis and pars distalis: effects of gonadotropin-releasing hormone and melatonin. Neuroendocrinology 66(4):263-70
Slanar, O. Zemkova, H. Vanecek, J. (1997) Melatonin inhibits GnRH-induced Ca2+ mobilization and influx through voltage-regulated channels. Biol Signals, 6:284-290.
Starkey, SJ Walker, MP Beresford, IJ Hagan RM (1995) Modulation of the rat suprachiasmatic circadian clock by melatonin in vitro. Neuroreport, 6:1947-1951.
Tang, PL Pang, SF Reiter, RJ (1996) Melatonin: A Universal Photoperiodic Signal with Diverse Actions. Karger, Basel.
Valenti, S Giusti, M Guido, R Giordano, G (1997) Melatonin receptors are present in adult rat Leydig cells and are coupled through a pertussis toxin-sensitive G-protein. Eur J Endocrinol, 136(6):633-9
Vanecek, J (1988) The melatonin receptors in rat ontogenesis. Neuroendocrinology, 48:201-203
Vanecek, J Pavlik, A Illnerova, H (1987) Hypothalamic melatonin receptor sites revealed by autoradiography. Brain Res, 435:359-362.
Wan, Q Liao, MX Pang, CS Pang, SF Brown, GM (1997) Guanosine 5'-O-(3-thiotriphosphate) and cations regulate melatonin receptors and melatonin inhibits cyclic AMP production in the spinal cord. Biol Signals 6:67-76
Wang, ZP Cheng, KM Brown, GM Pang, CS Pang, SF (1992) Characterization of 2-[125I]iodomelatonin binding sites in quail testes at midlight and middark. Neurosci Lett, 146:195-198.
Weaver, DR Reppert, SM (1996) The Mel1a melatonin receptor gene is expressed in human suprachiasmatic nuclei. Neuroreport, 8:109-112.
Williams LM Hannah LT (1995) High-affinity melatonin receptors are present on the epididymis of the immature rat. 77th Annual Meeting of the Ednocrinology Society, Washington, P3-404.
Wun, WSA. Jackson, FL Preslock, JP Perkowitz, AS (1986) Effect of melatonin in vivo upon FSH and LH release from hamster pituitary glands. Mol Cell Endocrinol, 46:227-234.
Yie, SM Niles, LP YoungLai, EV (1995) Melatonin receptors on human granulosa cell membranes. J Clin Endocrinol Metab, 80:1747-1749.
Song, Y Ayre, EA Pang, SF (1993) [125I]Iodomelatonin binding sites in mammalian and avian kidneys. Biol. Signals 2: 207-220
Yu, ZH Chow, PH Pang, SF (1994) Identification and characterization of 2[125I]iodomelatonin binding sites in the rat epididymis. J Pineal Res, 17:195-201.
Zhao, H Poon, AMS Pang SF (1998a) Putative melatonin receptors in the rat uterus: autoradiography and radioreceptor studies. Biol Signals Recept, 7:269.
Zhao, H Poon, AMS Pang SF (1998b) Localization and characterization of 2[125I]iodomelatonin binding sites in female rat uterus. FASEB J, 12(5): A686.
| Discussion Board | Previous Page | Your Symposium |