Invited Symposium: Pineal and its Hormone Melatonin
| INABIS '98 Home Page | Your Session | Symposia & Poster Sessions | Plenary Sessions | Exhibitors' Foyer | Personal Itinerary | New Search |
Immunomodulation By Melatonin
The functional relationship between the pineal gland and the immune system was initially suggested by the disorganization of thymic structures observed after pinealectomy (Px) of newborn rats. Px or any other experimental procedure that inhibits melatonin synthesis and secretion induces a state of immunodepression, counteracted by melatonin in several species (for ref. see [8-10]). In vivo, melatonin displays an immunoenhancing effect, particularly apparent in immunodepressive states. Melatonin administration augments antibody responses, such as plate-forming cell activity, cytotoxic T-cell responses and antibody dependent cellular cytotoxicity, corrects immunodeficiency that followed stress, immunosuppressant drugs or viral infections, and prevents apoptosis in hematopoietic cells and thymocytes.
Melatonin modulates in vitro proliferation of stem and precursor cells for granulocytes and macrophages in bone marrow. It also augments T-helper cell and natural killer (NK) activity, interleukin-2 and gamma-interferon production, antigen presentation and interleukin 1 mRNA production in human monocytes, as well as the expression of the proopiomelanocortin gene in lymph nodes and bone marrow. In vitro, inhibitory influences of melatonin on immune function are also found, e.g., inhibition of NK activity or of gamma-interferon synthesis [8-10].
Melatonin, in physiological concentrations, stimulated activated CD4+ T cells in vitro to release opioid agonist(s) with immunoenhancing and antistress properties [8]. As an indication of a local feedback loop, macrophages and monocytes appeared to synthesize melatonin upon gamma-IFN activation. Melatonin potentiated the activity of interleukin-2 in host antitumor responses in humans and was able to rescue bone marrow cells from apoptosis induced by antitumoral chemotherapeutic drugs in vitro and in vivo. These effects are probably on bone marrow T helper cells, by stimulating the production of interleukin-4 like substances [8-10].
The nature of the mechanisms involved in the immunomodulatory activity of melatonin remains unsettled. There is evidence suggesting the existence of membrane specific binding sites for melatonin in immune cells [11]. Nuclear melatonin receptors may also mediate melatonin immunomodulation, since drugs that bind to RZR/ROR receptors are active in experimental models of autoimmune diseases [12]. Melatonin is also a potent antioxidant, acting by itself rather than through specific binding sites [13]. In addition, melatonin may affect centrally hypothalamic hormone release [14].
During the last years we have examined the regulation of circadian rhythmicity of cell proliferation in rat submaxillary lymph nodes and spleen. In both immunized and non-immunized rats we reported a significant diurnal variation of submaxillary lymph node ornithine decarboxylase (ODC) activity (an index of cell proliferation in immune tissue), displaying maximal activity at early afternoon [4] and coinciding with maxima lymph node mitotic response to lipopolysaccharide and concanavalin A [5]. We also reported daily rhythms in splenic NK activity and lipopolysaccharide-induced cell proliferation which exhibited a maximum at midnight and at early morning, respectively, while concanavalin A-induced splenic proliferation peaked at midday [5].
Splenic ODC showed maxima at morning hours, which coincided temporally with changes in some related immune parameters (i.e., mitogenic activity) [6]. In our studies we observed that lymph node and splenic cell proliferation of Px rats decreased by about half still exhibiting a low amplitude, significant diurnal variations with a maximum at the afternoon. Administration of melatonin at late evening restored ODC levels and amplitude of diurnal rhythmicity [2,6]. The results were compatible with the view that the pineal gland plays a role in circadian changes of immune responsiveness in lymph nodes and spleen via an immunopotentiating effect of melatonin on cell proliferation. The interaction between the CNS and the immune system is a bi-directional process. Immunocompetent cells affect local neural processes by paracrine means through the release of cytokines and by endocrine means on the CNS via the bloodstream [15]. During the immune reaction there is an increase of sympathetic and parasympathetic activity in local nerves. Submaxillary lymph node and splenic sympathetic activity attained their maximum at early night, while cholinergic activity in lymph nodes peaked during the afternoon. After pineal removal a significant decrease in amplitude of rhythm of presynaptic activity in lymphoid organs was found. These sequela of Px were also counteracted by evening melatonin administration [2,6]. Therefore, melatonin may act in part by modifying circadian rhythmicity of neural signals conveyed to the immunocompetent organs via the autonomic nerves.
Circadian Immune Rhythms In Aging
Senescence is characterized by a decrease in the amplitude of circadian rhythms, as well as by alterations in entrainment to changes in the light-dark cycle o in non-photic stimuli (for ref. see [3]). In recent years we examined several aspects of the mycobacterial arthritis in rats as a model for studying the circadian organization of the immune responses during aging. Adjuvant-induced arthritis represents a T-lymphocyte-dependent autoimmune disease.
A study was carried out to examine whether 24-hour rhythms of ODC activity in lymph nodes and spleen and of presynaptic adrenergic and cholinergic markers, i.e. tyrosine hydroxylase (TH) activity and 3H-acetylcholine synthesis change during arthritis development and aging [3]. Our results were compatible with an aging-dependent, significant decrease of immune-mediated inflammatory response and of amplitude of 24-hour rhythms in cell proliferation and autonomic nervous system activity in lymph nodes and spleen.
One possible way age-dependent changes in circadian rhythmicity is produced is through age-dependent decrease in the activity of the autonomic nervous system. Recently we verified the existence of an aging-related, significant decrease in amplitude of 24-hour rhythms in autonomic nervous system activity, as assessed in selected autonomic ganglia and their innervating territories in rats by measuring TH activity and 3H-acetylcholine synthesis [16]. Some of the results obtained are depicted in Fig. 1-3.
Acrophases of 24-hour rhythms in norepinephrine and acetylcholine synthesis depended on the autonomic component tested. In pre- or paravertebral ganglia of the sympathetic nervous system, like the superior cervical, stellate or coeliac-superior mesenteric ganglia, and in the adrenal medulla (in a way, a modified sympathetic ganglion itself), acrophases of rhythms in 3H-acetylcholine synthesis, an indicator of the activity of preganglionic cholinergic terminals, coincided with those of TH activity, an index of the synthesis of norepinephrine transmitter in postganglionic sympathetic neurons. In each of these tissues both parameters exhibited peak activities at night (Fig. 1-3). In the hypogastric (main pelvic) ganglia, which are mixed ganglia containing both sympathetic and parasympathetic neurons receiving preganglionic sympathetic spinal input via the hypogastric (lumbar cord) and preganglionic parasympathetic input via the pelvic nerves (sacral cord) we found two rather than one daily maxima in 3H-acetylcholine synthesis, coinciding with the scotophase and the photophase of daily photoperiod. Since a maximum in TH activity occurred, as in other ganglia tested, at night, it seemed feasible that the scotophase peak in acetylcholine synthesis corresponded to activation of preganglionic lumbar cord terminals, while the photophase peak corresponded to preganglionic sacral cord terminals (Fig. 1 and 2). In the heart, acrophases of 24-hour rhythms in TH activity and 3H-acetylcholine synthesis did not coincide, TH peaking at night whereas 3H-acetylcholine synthesis peaking during the day (Fig. 3).
These results in selected autonomic territories are compatible with the sympathetic predominance during the activity phase, and the parasympathetic predominance during the resting phase, found during the day-night cycle in mammals [16]. For every of these rhythms examined, a decrease in amplitude with unmodified acrophases were found in aged rats. Therefore, our results, together with several studies measuring autonomic parameters at single time points underline a specific involvement of sympathetic, and to a less extent, parasympathetic dysregulation in senescent rats.
Among overt rhythms which have been shown to be of lower amplitude and/or phase-advanced in aging subjects is that of melatonin [17,18]. We recently reported an age-dependent, significant depression of pineal melatonin production in Sprague-Dawley rats [19]. We also observed that the effects of old age on circadian organization of the immune reaction were significantly counteracted by the evening administration of 10 or 100 micrograms of melatonin. Therefore, our observations are compatible with the view melatonin exerts a potent immunoenhancing activity in rats [19].
It must be noted that melatonin effect may not be always beneficial. Melatonin administration to young rats after mycobacterium or collagen injection induced a more severe arthritis than that expected in control animals [19]. Therefore, pharmacological levels of melatonin in young animals can overstimulate the immune system causing exacerbation of both autoimmune processes.
As shown in Fig. 1-3, treatment of rats with melatonin was effective, in old rats, to restore amplitude of daily rhythmicity in autonomic function [16]. The results were thus compatible with an age-dependent, significant effect of melatonin on autonomic neural activity.
 Click to enlarge
Fig. 1: Effect of melatonin on 24-hour changes in TH activity in sympathetic ganglia of old rats. Groups of 6-8, 18 months old rats were studied after receiving 17 daily s.c. injections of 10 or 100 micrograms of melatonin or its vehicle 1 h before lights off. Rats were killed at 6 different times throughout a 24-hour cycle (i.e., at 0900, 1300, 1700, 2100, 0100 or 0500 h). Also an additional group of rats was killed at 0900 h the next day. Shown are the means ± SEM. Melatonin at both doses restored amplitude of 24 hour rhythms. P values shown are those obtained in a factorial ANOVA. For further details see [16].
Click to enlarge
Fig. 1: Effect of melatonin on 24-hour changes in TH activity in sympathetic ganglia of old rats. Groups of 6-8, 18 months old rats were studied after receiving 17 daily s.c. injections of 10 or 100 micrograms of melatonin or its vehicle 1 h before lights off. Rats were killed at 6 different times throughout a 24-hour cycle (i.e., at 0900, 1300, 1700, 2100, 0100 or 0500 h). Also an additional group of rats was killed at 0900 h the next day. Shown are the means ± SEM. Melatonin at both doses restored amplitude of 24 hour rhythms. P values shown are those obtained in a factorial ANOVA. For further details see [16].
 Click to enlarge
Fig. 2: Effect of melatonin on 24-hour changes in 3H-choline conversion to acetylcholine in sympathetic ganglia of old rats. For details see legend to Fig. 1. Shown are the means ± SEM. Melatonin at both doses restored amplitude of 24 hour rhythms; in the case of hypogastric ganglia only the night peak was affected significantly by melatonin. P values shown are those obtained in a factorial ANOVA. For further details see [16].
Click to enlarge
Fig. 2: Effect of melatonin on 24-hour changes in 3H-choline conversion to acetylcholine in sympathetic ganglia of old rats. For details see legend to Fig. 1. Shown are the means ± SEM. Melatonin at both doses restored amplitude of 24 hour rhythms; in the case of hypogastric ganglia only the night peak was affected significantly by melatonin. P values shown are those obtained in a factorial ANOVA. For further details see [16].
 Click to enlarge
Fig. 3: Effect of melatonin on 24-hour changes in tyrosine hydroxylase activity and 3H-choline conversion into acetylcholine in heart and adrenal glands of old rats. For details see legend to Fig. 1. Shown are the means ± SEM. Melatonin at both doses restored amplitude of 24 hour rhythms. P values shown are those obtained in a factorial ANOVA. For further details see [16].
Click to enlarge
Fig. 3: Effect of melatonin on 24-hour changes in tyrosine hydroxylase activity and 3H-choline conversion into acetylcholine in heart and adrenal glands of old rats. For details see legend to Fig. 1. Shown are the means ± SEM. Melatonin at both doses restored amplitude of 24 hour rhythms. P values shown are those obtained in a factorial ANOVA. For further details see [16].
Other studies also underline the occurrence of restoring effects of melatonin on autonomic nervous system organization in aged animals. An age-related reduction in the maximal acetylcholine-induced contraction in the prostatic portion of rat vas deferens during the day was observed [20]. Both the nocturnal administration of melatonin and melatonin incubation of the vas deferens from old rats brought about a significant potentiation of the acetylcholine response.
For many years the idea that the pineal gland and melatonin were components of the mammalian circadian system was entertained with little experimental support. More lately, convincing data were provided that Px of rats leads to the increase in the rate of re-entrainment to a phase shift and that in constant light pineal removal causes a major disruption of the circadian system [21,22]. In view of the results depicted in Fig. 1-3, the hypothesis that melatonin, in addtion ot act directly on immune cells, augments amplitude, and perhaps, delays or advances the phase of the underlying central oscillator thus indirectly affecting time and quality of immune response, can be entertained.
Melatonin In Sleep Disorders
The sleep-promoting activity of melatonin in humans has been known since long [23,24]. More recently a number of studies pointed out a beneficial effect of melatonin in sleep disorders at an old age. Reversal of symptoms appears possible by increasing melatonin levels with either appropriately timed exposure to photic stimulation and/or appropriately timed administration of exogenous melatonin [25-27].
Among elderly people, even those who are healthy, there is an association of sleep disorders with impairment of melatonin production [25,26,28,29]. This is particularly marked in Alzheimer's disease (AD) patients [29,30]. As a part of a current project designed to to obtain information on the efficacy and safety of melatonin to treat sleep disorders in normal or pathological aging, we evaluate the efficacy of gelatin melatonin capsules on sleep in a geriatric population including patients with primary insomnia showing or not depression, and dementia patients with sundowning.
Twenty-eight females and 13 males (mean age 74 ± 12 years) were included in the first study [31]. Patients were divided into 3 groups for analysis: (a) patients with sleep disturbances alone (n= 22); (b) patients with sleep disturbance together with signs of depression (n= 9); (c) patients with sleep disorder and dementia of the degenerate (AD) or vascular type (n= 10). Patients were treated for 21 days with 3 mg-gelatin capsules of melatonin p.o. 30 min before expected sleeping time. Overall quality of sleep and daytime alertness were assessed from structured clinical interviews and from sleep logs filled by the patients or their caretakers. As an indirect estimation of agitated behavior at the beginning of the night, the coefficient variation of bed time was computed on days 0-2 and on days 19-21 of treatment.
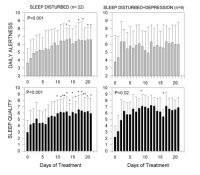
Forteen patients (8 females, 6 males) (72 ± 9 years) were included in the second study. AD was clinically diagnosed based on NINCDS-ADRDA Criteria. All patients were treated with melatonin (3 mg-gelatin capsules) in doses of 9 mg p.o.at bed time daily for variable periods, ranging from 22 to 35 months. Two of the patients who received 25 mg/day thioridazine because of the behavioral and sleep disorder interrupted thioridazine treatment after 5 and 24 months of starting melatonin treatment, respectively. Overall quality of sleep and daytime alertness were assessed from structured clinical and from sleep logs filled by caretakers. Neuropsychological evaluation of the patients was performed by the Functional Assessment Tool For AD (FAST) and Mini-Mental. Results were statistically analyzed by a non-parametric repeated-measures analysis of variance (ANOVA) followed by a Friedman's test, or by paired non-parametric Wilcoxon's test. Figure 4 shows the effect of melatonin treatment (3 mg dose) on daily alertness and quality of sleep in sleep-disturbed and sleep-disturbed, depressed patients. Although melatonin augmented subjective evaluation of quality of sleep in patients with sleep disturbances associated or not with depression symptoms, only in patients with sleep disturbances without any other major symptom melatonin augmented significantly morning freshness and daily alertness. Melatonin diminished significantly the number of interruptions of sleep both in patients with sleep disturbances alone and in those with sleep disturbances associated with depression symptoms.
 Click to enlarge
Fig. 4: Effect of melatonin treatment on subjective assessment of quality of alertness during the day and of quality of sleep. Groups of patients included: (a) patients with sleep disturbances alone (n= 22); (b) patients with sleep disturbance together with signs of depression (n= 9). Patients were treated from day 1 to day 21 with 3 mg capsules of melatonin, 30 min before expected sleeping time. Shown are the means ± S.D. Results were statistically analyzed by a non-parametric repeated-measures ANOVA followed by a Friedman's test. (*) p< 0.05, (**) p< 0.01 as compared to pretreatment value (modified from [9]).
Click to enlarge
Fig. 4: Effect of melatonin treatment on subjective assessment of quality of alertness during the day and of quality of sleep. Groups of patients included: (a) patients with sleep disturbances alone (n= 22); (b) patients with sleep disturbance together with signs of depression (n= 9). Patients were treated from day 1 to day 21 with 3 mg capsules of melatonin, 30 min before expected sleeping time. Shown are the means ± S.D. Results were statistically analyzed by a non-parametric repeated-measures ANOVA followed by a Friedman's test. (*) p< 0.05, (**) p< 0.01 as compared to pretreatment value (modified from [9]).
Table 1 summarizes the effect of melatonin treatment on coefficient of variation of bed time, an indirect estimation of agitated behavior at the beginning of the night. Such a coefficient averaged 58% in dementia patients as compared to 27-33% in patients not showing dementia, when estimated on days 0-2 of treatment. Only in dementia patients a significant decrease was detected on days 19-21 of treatment. In the case of dementia patients, a significant improvement of sundowning was clinically reported in 7 out of 10 patients studied. As far as the concomitant benzodiazepine treatment after completing melatonin treatment, 8 out of 13 patients with primary insomnia suppressed or reduced concomitant benzodiazepine use (by 50 to 75% of initial doses).
Table 1
Effect of melatonin treatment on variance of bed time (an indirect assessment of sundowning) in a geriatric, sleep-disturbed population of patients.
Insomnia (n=22) Insomnia+depression (n=9) Insomnia+dementia (n=10) --------------------------------------------------------------------- Days 0-2 Days 19-21 Days 0-2 Days 19-21 Days 0-2 Days 19-21 --------------------------------------------------------------------- 32.6 ± 14.9 31.5 ± 10.8 27.1 ± 14.1 24.5 ± 13.5 58.0 ± 24.7 41.5 ± 20.9* ---------------------------------------------------------------------
Patients were treated from day 1 to day 21 with 3 mg capsules of melatonin p.o., 30 min before expected sleeping time. Shown are the means ± S.D. of variation coefficients, i.e. [(S.D./mean) 100], of bed time values, as computed on days 0-2 and on days 19-21 of treatment . Results were statistically analyzed by a paired non-parametric Wilcoxon's test. (*) p< 0.03, as compared to 0-2 days value.
Results of the second open trial are summarized in Table 2. Neuropsychological evaluation at diagnosis of the 14 AD patients examined indicated a primary impairment of mnesic function of a varying degree in all subjects. All patients had cognitive and neuroimaging alterations (cortical and bitemporal atrophy) compatible with different evolutionary stages of AD. After varying periods of time of treatment with melatonin a significant improvement of sleep quality was found in all cases, the differences with the initial assessment being highly significant (p= 0.001, Wilcoxon's test) (Table 2).
Table 2
Effect of melatonin treatment (9 mg, p.o.) on subjective assessment of sleep quality and scores of FAST and Mini-Mental in 14 patients with AD.
# Age Sex Evolut. Treatm. Sleep Qual. FAST Mini-Mental
(months) (months) I F I F I F
-------------------------------------------------------------------
1 58 F 34 25 2 7 5 4 18 18
2 80 F 50 23 3 7 6a 5 15 15
3 76 F 70 30 2 8 7 6c 24 23
4 63 M 42 30 2 6 6a 5 24 23
5 77 M 48 23 2 5 5 4 20 19
6 78 F 65 30 4 6 3 2 19 19
7 68 M 38 25 2 5 4 3 24 25
8 68 F 46 22 3 6 4 4 19 19
9 82 F 70 30 3 6 6c 6c 5 5
10 53 M 80 24 2 5 6c 7a 2 2
11 77 F 30 22 3 6 2 2 24 25
12 66 M 82 24 2 5 6a 6c 9 9
13 69 F 72 30 3 5 4 5 9 9
14 80 M 70 35 2 8 6b 6b 10 10
-------------------------------------------------------------------
Patients received melatonin for 22 to 35 months. For every parameter assessed the initial (I) and present (F) value are given. Differences in sleep quality between initial and present assessment were significant (p= 0.001, Wilcoxon's test)
Sundowning, diagnosed clinically in all 14 patients examined, was not longer detectable in 12 of them, and persisted (although attenuated) in patients # 8 and # 9. Neuropsychological evaluation by FAST and Mini-Mental indicated absence of significant differences between initial and present state of evolution of the disease (Table 2). This should be contrasted with the significant deterioration of clinical conditions of the disease expected from patients after 1-3 years of evolution of AD (see e.g., [32]. No side effects of melatonin were detected in any of patients' population examined.
Changes in sleep-wake patterns are among the hallmarks of aging. During sleep there is an increase in the number and duration of waking episodes, a reduction in the nondreaming phases of sleep, the first REM phase occurs earlier in the night, and the tendency to fall asleep is increased during the day. Evidence indicates that since impaired melatonin secretion may be associated with sleep disorders in old age, the administration of melatonin can be helpful to treat sleep disorders in the elderly [25,26]. In a recent study we were able to demonstrated that the type of sleep augmented by melatonin in the elderly was mainly the non-REM sleep [33].
In the first study herein reported, an open, short-term, pilot examination of the sleep-promoting action of melatonin (3 mg p.o. for 21 days) was conducted in a small non-homogenous group of elderly patients with primary insomnia and with insomnia associated with depression or dementia [31]. The results indicated that melatonin therapy can be beneficial in the initiation and maintenance of sleep in patients suffering from insomnia alone or associated with depression symptoms. Melatonin efficacy to improve sleep did not correlate with a similar beneficial effect on mood in depressed patients. Melatonin was also hepful to reduce or suppress benzodiazepine comsumption, mainly in patients with primary insomnia.
Sundowning agitation is found in about half of the patients with dementia and is the most common cause of institutionalization of the demented geriatric patient [34]. In view that there was a clinical amelioration of sundowning in demented patients with the 3 mg melatonin dose used, but with relatively few effects on sleep quality, we decided to administer a higher melatonin dose (9 mg daily) to AD patients showing vesperal agitation. The retrospective account of these group of AD patients indicate that all patients in the present series treated with 9 mg melatonin daily did improve sleep quality and reduced or eliminated sundowning. So far, management of sundowning includes restriction of daytime sleep, exposure to bright light and social interaction schedules during the day [34]. The present results are strong evidence in favor of a beneficial effect of melatonin to treat sundowning.
Another important observation in the subjects studied was the halted evolution of the cognitive and mnesic alterations expected in comparable populations of patients not receiving melatonin. Although it cannot be stated at the present time whether this was a consequence of melatonin improvement in sleep-wake rhythms or an effect of melatonin treatment on any primary mechanism of AD, a putative interferring effect of melatonin in AD pathophysiology is not without experimental basis [35]. Indeed, in vitro data indicated that melatonin protects neurons against beta-amyloid toxicity [36,37], prevents beta-amyloid-induced lipid peroxidation [38] and alters the metabolism of the beta-amyloid precursor protein [39]. In a case report of two monozygotic twins suffering from AD of 8 years of evolution (treated one of them with melatonin, 6 mg p.o. for 36 months), evolution of the disease was halted in the melatonin-treated subject, as indicated by a stable impairment of mnesic function and a substantial improvement of sleep quality and reduction of sundowning [40]. Therefore, these results suggest that melatonin can be very helpful to treat AD patients.
This study was a part of a project supported by the University of Buenos Aires (TM 07), the Consejo Nacional de Investigaciones Científicas y Técnicas, Argentina (PIP 4156), the Agencia Nacional de Promoción Científica y Tecnológica, Argentina (A97B01 and PICT 2350) and Elisium S.A., Buenos Aires.
References
- Moore-Ede M: Physiology of the circadian timing system: predictive versus reactive homeostasis. Am J Physiol 1986;250:R737-R752
- Cardinali D P, Brusco L I, Selgas L, Esquifino AI: Melatonin: A synchronizing signal for the immune system. Neuroendocrinol Lett 1997;18:73-84
- Cardinali DP, Brusco LI, Selgas L, Esquifino AI: Diurnal rhythms in ornithine decarboxylase activity and norepinephrine and acetylcholine synthesis in submaxillary lymph nodes and spleen of young and aged rats during Freund's adjuvant-induced arthritis. Brain Res 1998;789:283-292
- Cardinali DP, Della Maggiore V, Selgas L, Esquifino AI: Diurnal rhythm in ornithine decarboxylase activity and noradrenergic and cholinergic markers in rat submaxillary lymph nodes. Brain Res 1996;711:153-162.
- Esquifino AI, Selgas L, Arce A, Della Maggiore V, Cardinali DP. Twenty four hour rhythms in immune responses in rat submaxillary lymph nodes and spleen. Effect of cyclosporine. Brain Behav Immunity 1996; 10:92-102
- Cardinali DP, Cutrera RA, Garcia Bonacho M, Esquifino AI: Effect of pinealectomy, superior cervical ganglionectomy and melatonin treatment on 24-hour rhythms in ornithine decarboxylase and tyrosine hydroxylase activities of rat spleen, J Pineal Res 1997;22:210-220.
- Esquifino AI, Cardinali DP: Local regulation of the immune response by the autonomic nervous system. Neuroimmunomodulation 1994;1:265-273
- Maestroni, GJM: T-helper-2 lymphocytes as a peripheral target of melatonin. J Pineal Res 1995;18:84-89
- Liebmann PM, Wolfer A, Felsner P, Hofer D, Schauenstein K: Melatonin and the immune system. Int Arch Allergy Immunol 1997;112:203-211.
- Fraschini F, Demartini G, Esposti D, Scaglione F: Melatonin involvement in immunity and cancer. Biol Signals Recept 1998;7:61-72.
- Poon AM, Pang SF: Pineal melatonin-immune system interactions; in: Tang PL, Pang SF, Reiter RJ (eds): Melatonin: A Universal Photoperiodic Signal with Diverse Actions, Basel, Karger, 1996, pp. 71-83.
- Missbach M, Jagher B, Sigg I, Nayeri S, Carlberg C, Wiesenberg I: Thiazolidine diones, specific ligands of the nuclear receptor retinoid Z receptor/retinoid acid receptor-related orphan receptor alpha with potent antiarthritic activity. J Biol Chem 1996;271:13515-13522.
- Reiter RJ: Functional pleiotropy of the neurohormone melatonin: antioxidant protection and neuroendocrine regulation. Front Neuroendocrinol 1995;16:383-415.
- Cardinali DP, Golombek DA, Rosenstein RE, Cutrera RA, Esquifino AI: Melatonin site and mechanism of action: Single or multiple? J Pineal Res 1997;23:32-39
- Besedovsky HO, Del Rey A: Immune-neuro-endocrine interactions: Facts and hypotheses. Endocr Rev 1996;17: 64-102.
- Brusco LI, García-Bonacho M, Esquifino AI, Cardinali DP: Diurnal rhythms in norepinephrine and acetylcholine synthesis of sympathetic ganglia, heart and adrenals of aging rats: Effect of melatonin. J Auton Nerv System in press.
- Arendt J: Melatonin and the Mammalian Pineal Gland, London, Chapman & Hall, 1995.
- Ferrari E, Magri F, Dori D, Migliorati G, Nescis T, Molla G, Fioravanti M, Solerte SB: Neuroendocrine correlates of the aging brain in humans, Neuroendocrinology 1995;61:464-474.
- Cardinali DP, Brusco LI, Garcia Bonacho M, Esquifino AI: Effect of melatonin on 24-hour rhythms of ornithine decarboxylase activity and norepinephrine and acetylcholine synthesis in submaxillary lymph nodes and spleen of young and aged rats. Neuroendocrinology 1998;67:349-362.
- Carneiro RC, Pereira EP, Cipolla Neto J, Markus RP: Age-related changes in melatonin modulation of sympathetic neurotransmission. J Pharmacol Exp Ther 1993;266:1536-1540.
- Armstrong SM: Melatonin and circadian control in mammals. Experientia 1989;45:932-939
- Cassone V: The pineal gland influences rat circadian activity rhythms in constant light. J Biol Rhythms 1992;7:27-40
- Vollrath L, Semm P, Gammel G: Sleep induction by intranasal administration of melatonin. Adv Biosci 1981;29:327-329.
- Waldhauser F, Saletu B, Trinchard Lugan I: Sleep laboratory investigations on hypnotic properties of melatonin. Psychopharmacol 1990;100:222-226.
- Garfinkel D, Laudon M, Nof D, et al. Improvement of sleep quality in elderly people by controlled-release melatonin. Lancet 1995;346:541-554
- Haimov I, Lavie P, Laudon M, et al. Melatonin replacement therapy of elderly insomniacs. Sleep 1995;18,598-603.
- Attenburrow MEJ, Cowen PJ, Sharpley AL: Low dose melatonin improves sleep in healthy middle-aged subjects. Psychopharmacology 1996;126:179-181.
- Iguchi H, Kato KI, Ibasayhi H: Age-dependent reduction in serum melatonin concentration in healthy human subjects. J Clin Endocrinol Met 1982;55:27-29.
- Skene DJ, Vivien-Roels B, Pevet P, et al. Daily variation in the concentration of melatonin and 5-methoxytryptophol in the human pineal gland: effect of age and Alzheimer disease. Brain Res 1990;528:170-174.
- Uchida K, Okamoto N, Ohara K, Morita Y: Daily rhythm of serum melatonin in patients with dementia of the degenerate type. Brain Res 1996;717:154-159
- Fainstein I, Bonetto AJ, Brusco LI, Cardinali DP: Effects of melatonin in elderly patients with sleep disturbance. A pilot study. Current Ther Res 1997;58:990-1000.
- Jost BC, Grossberg GT: The evolution of the psychiatric symptoms in Alzheimer's disease: A natural history study. J Am Geriatr Soc 1996;44:1078-1081
- Monti JM, Alvariño F, Cardinali D, Savio I, Pintos A: Effect of melatonin on sleep in elderly patients with chronic primary insomnia. Int Arch Geriatrics Gerontol, in press.
- McGaffigan S, Bliwise DL: The treatment of sundowning: A selective review of pharmacological and nonpharmacological studies. Drugs & Aging 1997;10:10-17.
- Reiter RJ: Oxidative processes and antioxidative defense mechanisms in the aging brain. FASEB J 1995;9:526-533.
- Pappolla MA, Sos M, Omar RA, Bick RJ, Hickson-Bick DL, Reiter RJ, Efthimiopoulos S, Robakis NK: Melatonin prevents death of neuroblastoma cells exposed to the Alzheimer amyloid peptide. J Neurosci 1997;17:1683-1690.
- Pappolla M, Bozner P, Soto C, Shao H, Robakis NK, Zagorski M, Frangione B, Ghiso J: Inhibition of Alzheimer b-fibrillogenesis by melatonin. J Biol Chem 1998;273:7185-7188.
- Daniels WM, Van Rensburg SJ, Van Zyl JM, Taljaard JJ: Melatonin prevents b amyloid, induced lipid peroxidation. J Pineal Res 1998;24:78-82.
- Song W, Lahiri K: Melatonin alters the metabolism of the b-amyloid precursor protein in the neuroendocrine cell line PC12. J Mol Neurosci 1997;9:75-92.
- Brusco LI, Márquez M, Cardinali DP: Monozygotic twins with Alzheimer's disease treated with melatonin. Case report. J Pineal Res, in press.
| Discussion Board | Previous Page | Your Symposium |