Invited Symposium: Pineal and its Hormone Melatonin
| INABIS '98 Home Page | Your Session | Symposia & Poster Sessions | Plenary Sessions | Exhibitors' Foyer | Personal Itinerary | New Search |
Introduction
The pineal hormone melatonin has been implicated in the regulation of circadian rhythmicity and of seasonal reproductive responses in mammals. The biological functions of melatonin are mediated by melatonin receptors. Although the majority of the previous research on melatonin receptors has focused on the suprachiasmatic nucleus (SCN) and pars tuberalis (PT) as the major brain targets of melatonin, accumulating evidence indicates a more widespread distribution of the receptors in the brain(1). The retina is another source of melatonin in the CNS, but the hormone acts locally, ctivating its receptors located within the tissue(2). The precise localization of melatonin receptors in the brain and retina is still poorly understood. Two melatonin receptor subtypes, Mel1a and Mel1b have been cloned in mammals(3,4). Although both subtypes are linked to the inhibition of adenylyl cyclase, these receptors exhibit distinct expression patterns in the mammalian CNS, suggesting that the different subtypes may mediate different melatonin effects in the CNS. Yet the role of each receptor subtype in the diverse biological activities of melatonin remains unclear. Several studies implicate SCN GABAergic transmission in the generation and/or modulation of circadian rhythmicity(5,6). Thus, we hypothesize that melatonin may regulate GABAA receptor function in the SCN and thereby exert its effects on circadian rhythmicity. The goals of the present study are 1) to localize the Mel1a receptor in rat brain and retina and 2) to reveal the modulatory role of melatonin on GABAA receptor function.
Materials and Methods
Anti-Mel1a receptor antibody was raised against a synthetic peptide corresponding to the third intracellular loop of the human Mel1a receptor (7). Western blots of the Mel1a receptor were performed in rat brain regions and retina. Immunocytochemistry for Mel1a was carried out in rat retina. Localization of Mel1a mRNA in rat retina was examined by in situ hybridization using digoxigenin-labeled cRNA probes.
Standard whole-cell recordings were performed in SCN and
CA1 neurons of adult rat hypothalamic and hippocampal slices, as well as
in transfected HEK 293 cells. GABAA receptor-mediated whole-cell currents
were induced by pressure-ejection of GABA. RT-PCR analysis was performed
to examine the mRNA expression of Mel1a or Mel1b in rat SCN and hippocampal
tissues, and in transfected HEK 293 cells.
Western blotting of Mel1a receptor in the rat brain revealed
a single immunoreactive band at approximately 37kD in all regions examined,
i.e., cerebellum, medulla, midbrain, neocortex and hypothalamus (Fig. 1).
The control blots which were treated with the antibody preabsorbed with
the immunogen peptide showed no immunoreactive band.
Fig. 1: Western blots of the Mel1a receptor in discrete
regions of rat brain. Western blotting of the Mel1a receptor in the rat retina showed a
37kD band as well, which was blocked with immunogen peptide.
Immunocytochemistry revealed a specific immunoreaction in the inner
plexiform layer and the outer plexiform layer of the rat retina. Mel1a
mRNA was localized to ganglion cells, amacrine cells and horizontal cells
by in situ hybridization using antisense RNA probes. No hybridization signals
were obtained with sense RNA probes. To test the effects of melatonin on
GABAA receptor function in SCN, we have used whole-cell patch-clamp techniques
to record GABAA receptor-mediated currents in SCN neurons of rat hypothalamic
slices. Neurons located in the ventrolateral portion of the nucleus were
recorded under voltage-clamp mode at a holding potential of -60 mV (Fig.
2A). Pressure ejection of GABA (10 mM) toward the neuron induced inward
currents. Bath application of melatonin (1 nM) increased current amplitudes
in 19 out of 30 cells tested (2893±198 pA for melatonin treatment
versus 2268±216 pA for control; Student's t test, p < 0.05) with
no effects on the remaining cells ( Fig 2B), suggesting that activation
of melatonin receptors in the majority of these neurons can up-regulate
GABAA receptor function.
To determine whether the enhancement of GABAA currents by melatonin
is unique to neurons in the SCN or generalized to neurons in the central
nervous system, we next investigated effects of melatonin on GABAA receptor-mediated
currents in CA1 neurons in hippocampal slices (Fig. 2D). To our surprise,
we did not observe melatonin-induced enhancement of the GABAA receptor-mediated
currents in any neurons tested in this region. Melatonin treatment was
instead found to decrease current amplitudes in 17 out of 25 CA1 neurons
tested ( 1206±96 pA in melatonin treatment versus 1591±153
pA in control recording; p < 0.05; Fig. 2E). Thus, the effect of melatonin
on GABAA receptor function in the responsive hippocampal neurons is opposite
to that in responsive SCN neurons. Among many possibilities, the simplest
explanation is that the SCN and the hippocampus may express different melatonin
receptor subtypes which may mediate the opposite effects of melatonin on
GABAA receptor function. Thus, we performed RT-PCR using primers specific
for rat Mel1a and Mel1b to analyze the expression of Mel1a or Mel1b mRNA
in rat SCN and hippocampus. As shown in Figure 2C, 2F, PCR products from
the SCN specifically hybridized with the rat Mel1a oligonucleotide probes
and in contrast, the hippocampal products were only recognized by the Mel1b
probes. Subsequent cDNA subcloning and sequencing confirmed the identity
of the products amplified from SCN tissue as rat Mel1a fragment and that
from the hippocampus as rat Mel1b receptor fragment.
The opposite modulation of GABAA receptor function by melatonin in
conjunction with the differential expression of Mel1a and Mel1b receptors
in the SCN and hippocampus strongly suggests that Mel1a and Mel1b receptors
may have distinct roles in modulating GABAA receptor function.
To test this hypothesis directly, we transiently co-transfected GABAA
receptor a1b2g2 subunits with either Mel1a or Mel1b receptors into HEK293
cells. Overexpression of the Mel1a or Mel1b receptor genes in these cells
was confirmed by RT-PCR analysis. We found that melatonin (1 nM) had no
detectable effect on GABAA receptor-mediated whole-cell currents in cells
expressing recombinant GABAA receptors only (1521±159 pA in control
recording, 1510±154 pA in melatonin treatment; p 0.05, n = 6; Fig.
2G). However, melatonin (1 nM) increased the GABAA currents in cells expressing
Mel1a receptors (1502±86 pA in control recording, 1818±112
pA in melatonin treatment; p < 0.05, n = 9; Fig. 2G), whereas it reduced
the currents in cells expressing Mel1b receptors (1602±75 pA in
control recording, 1265±68 pA in melatonin treatment; p < 0.05,
n = 6; Fig. 2G). Thus these data confirm that the two melatonin receptor
subtypes can mediate opposite effects on GABAA receptor function.
We have demonstrated expression of the Mel1a receptor
protein in a variety of regions in rat brain. The physiological significance
of melatonin receptors in those several brain regions is still not known.
The widespread distribution of melatonin receptors shown in this study
provides evidence for the diverse physiological functions of the hormone
in the central nervous system.
Results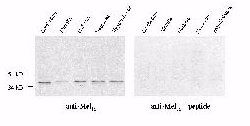
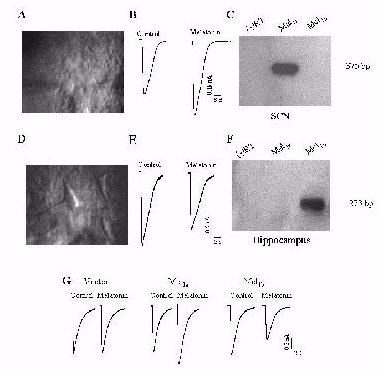 Fig. 2: (A) A
high magnification infrared DIC video image of rat SCN neurons.(B) Melatonin
(1 nM) potentiates GABAA receptor-mediated whole-cell currents in a SCN
neuron. (C) RT-PCR analysis of Mel1a gene expression in rat SCN. (D) A
high magnification infrared DIC video image of rat hippocampal CA1neurons.
(E) Melatonin (1 nM) inhibits GABAA receptor-mediated whole-cell currents
in a CA1 neuron. (F) RT-PCR analysis of Mel1b gene expression in rat hippocampus.
(G) Melatonin (1 nM) has no effect on GABA current in cells transfected
with a1b2g2 (p 0.05, n =6), but enhances GABA current in cells transfected
with the a1b2g2/Mel1a combination (p < 0.05, n =9) and inhibits GABA
currents in cells transfected with a1b2g2/Mel1b (p < 0.05, n =6).
Fig. 2: (A) A
high magnification infrared DIC video image of rat SCN neurons.(B) Melatonin
(1 nM) potentiates GABAA receptor-mediated whole-cell currents in a SCN
neuron. (C) RT-PCR analysis of Mel1a gene expression in rat SCN. (D) A
high magnification infrared DIC video image of rat hippocampal CA1neurons.
(E) Melatonin (1 nM) inhibits GABAA receptor-mediated whole-cell currents
in a CA1 neuron. (F) RT-PCR analysis of Mel1b gene expression in rat hippocampus.
(G) Melatonin (1 nM) has no effect on GABA current in cells transfected
with a1b2g2 (p 0.05, n =6), but enhances GABA current in cells transfected
with the a1b2g2/Mel1a combination (p < 0.05, n =9) and inhibits GABA
currents in cells transfected with a1b2g2/Mel1b (p < 0.05, n =6).
Discussion and Conclusion
Mel1a receptor was immunocytochemically localized to the inner and outer plexiform layers and Mel1a receptor transcripts were localized to ganglion, amacrine and horizontal cells. These results suggest that melatonin influences retinal physiology by acting on these retinal cell types via the Mel1a receptor expressed in their processes, located in the inner and outer plexiform layers.
The lack of subtype-specific agonists and antagonists
of melatonin receptors has made it impossible to determine the contributions
of these two receptor subtypes to melatonin actions in the mammalian CNS.
In the present work, we have provided strong evidence that these two receptors
can mediate opposite effects of melatonin on a given biological function,
suggesting that the receptor subtypes are critical determinants of the
diversity of melatonin actions in the mammalian brain. GABA is the major
inhibitory neurotransmitter in the mammalian SCN. In light of recent evidence
indicating that GABAA receptor-mediated neurotransmission plays a pivotal
role in the generation of cyclic firing activity of the SCN neurons, and
since melatonin can act directly at the SCN to inhibit neuronal firing,
the enhancement of GABAA receptor function by melatonin, through Mel1a
receptors, may be responsible for the regulatory effects of melatonin on
mammalian circadian time-keeping and melatonin's sleep-inducing effects.
In addition, our results demonstrate that melatonin, through its Mel1b
receptors, may directly affect mammalian hippocampal function.
References
Back to the top.
| Discussion Board | Previous Page | Your Symposium |