Biomedical Education Poster Session
| INABIS '98 Home Page | Your Session | Symposia & Poster Sessions | Plenary Sessions | Exhibitors' Foyer | Personal Itinerary | New Search |
Introduction
Potentials from the primary somatosensory cortex are recorded on scalp electrodes following electrical stimulation of peripheral nerve trunks. The earliest of these somatosensory evoked potentials (SEPs) can be modulated by cortical activity (Candedo 1997) and as a consequence of discharge from peripheral sensory receptors activated by movement (Brooke et al. 1997). The primary potential is also modulated when accuracy is a requirement of the task (Staines et al. 1997). Presently, it is not known how the primary or secondary components of the SEP alter during the acquisition of a motor task.
We are constantly using sensory cues from the environment. However, the use of such cues depends on our knowledge and familiarity of the surrounding environment and of our ongoing activity. Practice of a motor task can refine the integration of novel external cues into successful task performance. Currently, it is not clear how the gain of an ascending sensory path alters as a skill is acquired which involves an external cue transmitted over that path. Gain refers to the change in magnitude of the recorded potential for a constant stimulus input. Specifically, does the gain of a cutaneous path from the lower limb alter during the learning of a motor task which requires the transmission of an external cue through that pathway? The focus of the present study was to investigate the modulation of a cutaneous path (from sural nerve), during the initial acquisition of a motor skill compared to when the task has been well learned.
We hypothesized a gain attenuation of the primary (P1-N1) SEP when the task had been successfully 'acquired'. The task required subjects to plantar flex to a target 150 beyond a cue (1) fixed in one position (2) occurring at one of three locations-variable
Materials and Methods
Subjects 8 volunteer subjects of both genders (ages 20 to 26 years) gave informed consent prior to their participation in the study and none reported any history of neurological, cardiovascular and musculoskeletal deficits. The experimental procedures were approved by the University of Guelph Ethics Committee for experiments on humans. Subjects reported for one, three hour session.
Stimulation Procedures
The sural nerve on the left leg was stimulated with a 0.5ms (Grass S88 stimulator with SIU5A Stimulus Isolation Unit, Grass Inst. Co., Mass., USA) square wave pulse posterior and proximal to the lateral malleolus, with anode distal. Stimulus intensity was maintained at 2-3 times perceptual threshold and monitored before, mid and post-experiment. This stimulus was used to elicit the SEP. A second stimulus (SD9 stimulator, Grass Inst.) acted as an external cue during the experiment and was delivered to the lateral border of the left foot.
Recording Procedures
SEPs were recorded from Cz' (2 cm caudal to Cz) and referenced to Fpz' (2 cm caudal to Fpz) in accordance with the International 10-20 System. Scalp electrodes in an Electro-Cap System (ECI) were used for all SEP recordings. Impedance at all recording sites was less than 3kohms (EZM5 impedance meter, Grass Inst. Co., Mass., U.S.A.). Electromyographic (EMG) recordings were made from the left soleus muscle. Ag/AgCl surface electrodes were oriented approximately 2 cm apart longitudinally over the predicted path of muscle fibres. Impedance at all EMG recording sits was less than 10kohms. The evoked potentials and EMG recordings were amplified (40,000X, 1000X, respectively) and filtered (band-pass 1-100Hz, 3-300Hz, respectively) with an isolated bioelectric amplifier (SA Instruments, California, U.S.A.). Data were sampled at 1000Hz (Keithley DAS-1800 HC) by a microcomputer for 150ms for each sample, initiated 30ms prior to the stimulus. Each SEP trace was the average of 30 to 40 individual samples visually inspected to be artifact free.
Procedure
Subjects were seated with head and back supported in a reclined customized chair. Opaque goggles eliminated visual input and noise was reduced to a minimum. Each foot was firmly braced to a foot pedal which rotated the ankle about its axis. Beginning each trial in a fully dorsi- flexed position, subjects began plantar flexing their left foot at a self-paced velocity. Within the first 150 of plantar flexion, the nerve was stimulated, evoking the cortical somatosensory potential and muscle reflex. Subjects continued plantar flexing in search of the second stimulus, the external cue which occurred on the lateral border of the foot (See Figure 1). Once receiving the cue, subjects were to move a further 150 and stop when they decided they had reached this point, the target. Subjects received auditory feedback (tone) based on the accuracy of their target acquisition, as too short (greater than 1.50 before the target), perfect (+/- 1.50 of the target) or too long (greater than 1.50 beyond the target). The tone signalled the end of each trial.
Conditions were (1) pre and post-movement stationary control (2) Practice, Fixed (the cue always occurred in the same spatial location in each trial) (3) Acquired, Fixed (same as #2 however subjects had successfully learned the task (above 70% accuracy)) (4) Practice, Variable (the cue occurred at one of three locations spaced 150 apart and chosen for each sample) (5) Acquired, Variable (same as #4 however subjects had successfully learnt the task). Subjects were aware of which experimental condition they were in. ....as can be seen in Figure 1.
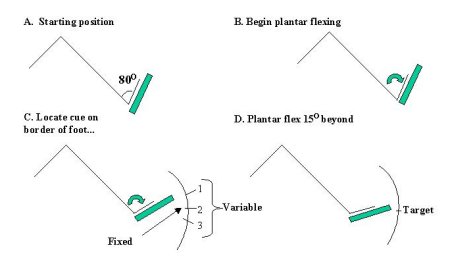 Fig.1: The Task
Fig.1: The Task
Data Analysis
SEP traces with artifacts were rejected by visual inspection so that approximately 30-40 artifact- free samples were averaged per subject-condition. Amplitudes and latencies were measured from each averaged SEP trace. Amplitude measurements were taken from eh peak of the first positive waveform to the peak of the subsequent negative deflection (P1-N1) and from the peak of the second positive waveform to the negative peak following (P2-N2).
...as can be seen in Figure 2
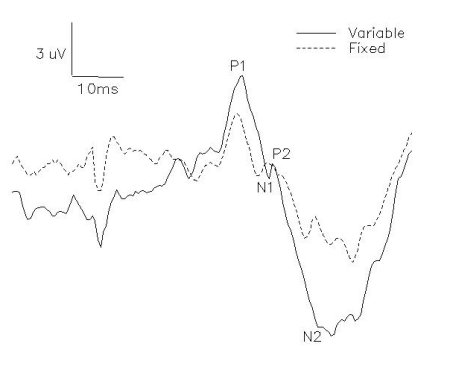 Fig. 2: P1-N1 and P2-N2 amplitudes were recorded from Cz' and referenced to Fpz'.
Fig. 2: P1-N1 and P2-N2 amplitudes were recorded from Cz' and referenced to Fpz'.
The statistical analysis for magnitudes of SEP, accuracy and area of pre-stimulus EMG used a one-factor (experimental conditions) analysis of variance blocked on subjects for all experiments. The research hypotheses were tested with a priori comparisons ( t-tests with the Bonferroni correction) which utilized the error rms from the analysis of variance (Keuhl 1994). Significance was set at p<.05.
Results
Mean latencies (with standard deviations) of P1, N1, P2 and N2 over all subjects were 47.57+/- 3.34, 59.92+/-3.63, 64.78+/-3.27, 78.09+/-9.22, respectively. The intensity of sural nerve stimulation was maintained between 2-3 perceptual threshold. The stationary controls, sampled prior to an after movement conditions, did not differ significantly for any of the subjects in any experiments. The following results in each experiment are from the a priori comparisons between the task conditions carried out over eight subjects.
The effect of initial practice on SEPs elicited from sural nerve stimulation, the channel transmitting the cue As is seen in Figure 2A and 2C, the mean primary SEP waveform was significantly attenuated, by 34% and 35% respectively, in the acquired versus the practice condition for the fixed and variable cued tasks [F(1,35)=12.3, 10.9, p<.05]. Vertical bars show standard errors. Pre-stimulus EMG did not alter significantly in either task (p>.05). Target acquisition improved significantly from the practice to the acquired condition for both tasks [F(1,21)=13.5, 15.2, p<.05] (Figure 2B and 2D). The secondary waveform did not alter significantly with prolonged exposure for the fixed or variable cued task (Figures 2B, 2D).
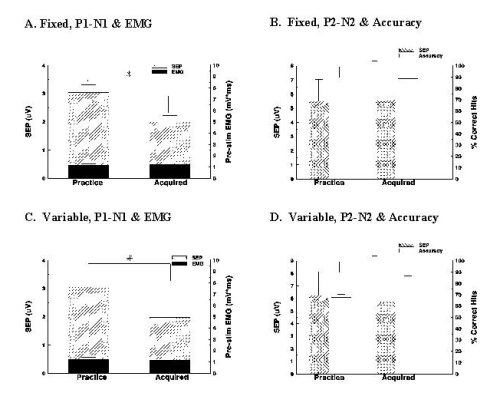 Fig. 3: Results
Fig. 3: Results
Discussion and Conclusion
The research hypothesis was supported by the results. Primary SEP magnitudes were depressed once the fixed or variable cued motor task had been successfully acquired. In addition to the testing of the hypothesis, it was observed that the accuracy of target acquisition improved, being almost 90% when the cue was well acquired. There was no observable modulation of the secondary, P2-N2 complex.
Clearly, task acquisition resulted in the depression of primary SEPs. The site of this modulation of the SEPs could be located at any of the synapses in the ascending path, including the cortical receptive cells themselves (Homberg and Huttenen 1991, Jueptner et al. 1997, Eguibar et al. 1997). Practice did not yield any modulatory effect on the magnitude of the secondary complex. There is no consensus of opinion as to the origin of the secondary complex of the SEP. It may arrive from the large diameter afferents through an additional brain site before arrival at the cerebral cortex (Redmond 1975).
It is unlikely that the present results arose from technical deficiencies in the conduct of the experiment. Instructions to subjects were standardized. Stimulus intensity was controlled by evaluating perceptual thresholds and the comparability of ongoing EMGs in soleus was checked across conditions. Movement velocity was constant over the cued conditions. The subjects resisted boredom, as evidenced by the maintained accuracy of target acquisition.
Sensory gating has been observed in other members of the vertebrate and invertebrate animal kingdom (Prochazka 1989, Watson 1992). Gating or facilitation of sensory input may serve to dampen or enhance essential afferent signals during ongoing activity. The depressed gain observed during task acquisition provides an example of the how sensory input is modified into appropriate behaviour.
References
- Brooke JD, Cheng J, Collins DF, McIlroy WE, Misiaszek JE, Staines WR (1997) Sensori- sensory afferent conditioning with leg movement: gain control in spinal reflex and ascending paths. Prog Neurobiol 51: 393-421.
- Candedo A (1997) Primary motor cortex influences on the descending and ascending systems. Prog Neurobiol 51: 287-355.
- Eguibar JR, Quevedo J, Rudomin P (1997) Selective cortical and segmental control of primary afferent depolarization of simgle muscle afferents in the cat spinal cord. Exp Brain Res 113(3):411-430.
- Huttenen J, Homberg V (1991) Modification of cortical somatosensory evoked potentials during tactile exploration and simple active and passive movements. Electroencephalogr Clin Neurophysiol 81(3): 216-223.
- Jueptner M, Frith CD, Brooks DJ, Frackowiak Rs, Passingham RE (1997) Anatomy of motor learning. II. Subcortical structures and learning by trial and error. J Neurophysiol 77(3):1325- 1337.
- Prochazka A, Trend P, Hulliger M, Vincent S (1989) Ensemble proprioceptive activity in the cat step cycle: towards a representative look-up chart. Prog Brain Res 80:61-74.
- Redmond DE, Borge GF, Buchsbaum M, Mass JW (1975) Evoked potential studies of brain catecholamine alterations in monkeys. J Psychiatr Res 12(2) 97-116.
- Staines WR, Brooke JD, Cheng J, Misiaszek JE, MacKay WE (1997a) Movement-induced gain modulation of somatosensory potentials and soleus H-reflexes evoked from the leg I. Kinaesthetic task demands. Exp Brain Res 115: 147-155.
- Watson DHD (1992) Presynaptic moduolation of sensory afferents in the vertebrate and invertebrate nervous system. Comp Biochem Physiol 103(A):227-239.
| Discussion Board | Previous Page | Your Poster Session |