Invited Symposium: Neural Substrates of Sexual Motivation and Performance as Revealed by Neural Immediate-Early Gene Expression
| INABIS '98 Home Page | Your Session | Symposia & Poster Sessions | Plenary Sessions | Exhibitors' Foyer | Personal Itinerary | New Search |
Background
During the estrous cycle of female rats, sexual behavior is dependent upon an increase, first in estradiol levels, and then in progesterone. Estrous behavior is abolished by ovariectomy and reinstated by the sequential treatment of estradiol followed by progesterone.
A great deal of work that has been done over the past twenty years supports the idea that intracellular, neuronal progestin receptors (PRs), acting as transcription factors, are essential in mediating the effects of progesterone on sexual behaviors (e.g., 5, 6).
While appropriate underlying hormonal conditions are critical for the display of sexual receptivity, mating stimulation provided by the male also influences sexual behavior. One important stimulus provided by the male is vaginal-cervical stimulation (VCS). VCS elicits many different behavioral and endocrine changes in female rats, such as increases in lordosis, pseudopregnancy, and termination of sexual receptivity.
In an attempt to identify those neurons influenced by mating stimulation, we (8) and others used immunocytochemistry for immediate early proteins, including Fos. Female rats were either mated with male rats or administered VCS via a plastic probe. While mating stimulation induces Fos expression in a variety of neuroanatomical areas, the results suggest that much of the expression can be accounted for by one aspect of the stimulus - VCS. In general, the following areas showed increases in expression of Fos-IR after these stimuli: medial preoptic area, bed nucleus of stria terminalis, posterodorsal medial amygdala, dorsomedial hypothalamus, ventromedial hypothalamus, medial tuberal area and the midbrain central gray.
As VCS dramatically influences reproductive behavior, and reproductive behavior is mediated in part by steroid receptor-containing neurons, it is possible that VCS directly influences steroid receptor containing-neurons. Our first experiment (9) on this demonstrated that the majority of the neurons in which VCS induced Fos expression in the medial preoptic area, posterodorsal medial amygdala and bed nucleus of the stria terminalis also contained estrogen receptor-immunoreactivity. This demonstrates that hormonal and information relevant to genital stimulation may be integrated within specific neurons.
Progesterone induces Fos expression
While it was clear from many studies that mating stimulation could induce Fos expression in a variety of forebrain and midbrain areas, studies on the ability of progesterone to induce Fos expression were more controversial. In studies of the neuronal sites at which progesterone induces changes in Fos expression in ovariectomized, estradiol-primed rats, we found increases in the medial amygdala, medial preoptic area, dorsomedial hypothalamus, and portions of the ventromedial hypothalamus (Figure 1; ref. 3).
 Click to enlarge
Figure 1: Number of Fos-IR cells counted bilaterally for each area from vehicle (Oil + oil), estradiol alone (EB + Oil) or estradiol plus progesterone (EB + P) treated animals. MeA = medial amygdala, MPO = medial preoptic area, DMH = dorsomedial hypothalamus, VMH = ventromedial hypothalamus, L-VMH = area lateral to the VMH containing a high concentration of PR-IR neurons
Click to enlarge
Figure 1: Number of Fos-IR cells counted bilaterally for each area from vehicle (Oil + oil), estradiol alone (EB + Oil) or estradiol plus progesterone (EB + P) treated animals. MeA = medial amygdala, MPO = medial preoptic area, DMH = dorsomedial hypothalamus, VMH = ventromedial hypothalamus, L-VMH = area lateral to the VMH containing a high concentration of PR-IR neurons
We then attempted to determine if these neurons in which progesterone induces Fos also contain PRs, which might mediate the Fos response. We observed that many of the neurons in the medial preoptic area and ventromedial nucleus of the hypothalamus and its associated steroid receptor-rich area, as well as the arcuate nucleus contained progestin receptor-immunoreactivity (PR-IR). Because of limitations of the immunocytochemical procedure, we were not able to examine colocalization in other areas. Furthermore, these limitations result in a gross underestimate of the true colocalization (Figure 2; ref. 2).
 Click to enlarge
Figure 2: Mean number of Fos-IR cells containing PR-IR following treatment with estradiol benzoate + oil or estradiol benzoate + progesterone. MPO = medial preoptic area, rVMHVL = rostral ventrolateral aspect of the ventromedial nucleus of the hypothalamus, rVMHVL-ORA ovarian steroid receptor-rich area associated with the rVMHVL, cVMHVL = caudal VMHVL, Arc = arcuate nucleus.
Click to enlarge
Figure 2: Mean number of Fos-IR cells containing PR-IR following treatment with estradiol benzoate + oil or estradiol benzoate + progesterone. MPO = medial preoptic area, rVMHVL = rostral ventrolateral aspect of the ventromedial nucleus of the hypothalamus, rVMHVL-ORA ovarian steroid receptor-rich area associated with the rVMHVL, cVMHVL = caudal VMHVL, Arc = arcuate nucleus.
VCS effects on PR-IR neurons
As intraneuronal PRs have a central role in regulating sexual behaviors, mating stimulation could influence sexual receptivity via PRs. To test this idea, Fos immunocytochemistry was used to determine if neurons influenced by afferent input from the social environment, by neurotransmitters, or by hormone injection also contain PRs.
Mating stimulation, like progesterone, induces Fos expression in a variety of neuroanatomical areas. A subset of the Fos-expressing neurons also contains PRs, suggesting the presence of a population of neurons in which mating stimulation may influence PRs (Figure 3; ref. 4).
 Click to enlarge
Figure 3: Mean number of Fos-IR cells following VCS or control stimulation which also contained PR-IR. Abbreviations same as Figure 2
Click to enlarge
Figure 3: Mean number of Fos-IR cells following VCS or control stimulation which also contained PR-IR. Abbreviations same as Figure 2
These experiments demonstrate that hormonal information and afferent input from genital stimulation converge in particular neurons in the forebrain and midbrain. These Fos-PR colocalization studies enable us to study the cellular processes by which afferent input influences PR-containing neurons. It is in these neurons that response to afferent input and to progesterone may be integrated.
As earlier work suggested that dopamine, acting through D1 receptors, activates PRs in the brain, we tried to determine if dopamine might directly interact with PR-containing neurons. We infused the D1 receptor agonist, SKF 38393 into the cerebral ventricles, and immunostained brain sections for Fos-IR. We observed a population of neurons that expresses Fos in response to stimulation with a D1 agonist in areas containing an abundance of PR-IR neurons. Furthermore, a subpopulation of PR-IR neurons respond to the agonist, suggesting that dopamine and progesterone may act in some of the same neurons (Figure 4; ref. 6).
 Click to enlarge
Figure 4: Mean number of PR-IR cells that express Fos one hour after icv infusion of 100 ng of the D1 dopaminergic agonist, SKF 38393. Abbreviations are as defined in Figure 2.
Click to enlarge
Figure 4: Mean number of PR-IR cells that express Fos one hour after icv infusion of 100 ng of the D1 dopaminergic agonist, SKF 38393. Abbreviations are as defined in Figure 2.
VCS induces Fos via PRs in some neurons
In order to determine if VCS-induced Fos expression relies upon the PRs that we observed in some of the neurons, we attempted to block VCS-induced Fos by pretreatment with the progestin antagonists, RU 486 or ZK 98299 (3).
Ovariectomized female rats were administered VCS or control stimulation. One group of animals was administered the progestin antagonist prior to VCS. As predicted, injection of either of the progestin antagonists partially blocked the VCS-induced Fos expression in some brain areas, but not others (Figure 5). This effect was independent of adrenal progesterone, as similar results were obtained in ovariectomized/adrenalectomized rats as well.
 Click to enlarge
Figure 5: Number of Fos-IR cells per section for each area from estradiol-primed ovariectomized rats receiving manual VCS or control perineal stimulation. Rats received either 5 mg RU 486 or oil vehicle one hour prior to stimulation, and they were perfused one hour later. In addition to abbreviations defined in Figure 2, BSTM = bed nucleus of stria terminalis, PVA = anterior-periventricular thalamic nucleus.
Click to enlarge
Figure 5: Number of Fos-IR cells per section for each area from estradiol-primed ovariectomized rats receiving manual VCS or control perineal stimulation. Rats received either 5 mg RU 486 or oil vehicle one hour prior to stimulation, and they were perfused one hour later. In addition to abbreviations defined in Figure 2, BSTM = bed nucleus of stria terminalis, PVA = anterior-periventricular thalamic nucleus.
These results suggest that the VCS-induced Fos expression in some cells is dependent upon the availability of unoccupied PRs. They are consistent with the idea that some information provided by VCS activates neuronal PRs in the absence of progesterone. This suggests a process by which mating stimulation, in particular VCS, can directly influence PR function, and the expression of PR-dependent gene products.
Integration Via PRs
Progesterone induces Fos expression in some neurons; some of these progesterone-responsive neurons also contain PRs.
VCS-induces Fos expression in many neurons; many of these VCS-responsive neurons also contain PRs.
These experiments have enabled us to identify neurons in which integration of information from the social environment and the internal hormonal milieu may occur resulting in in cellular reponses that may be influenced by the interaction of both, afferent inputs and progestins.
The induction of Fos expression by VCS is blocked by a progesterone antagonist in some neurons, suggesting that afferent input may actually act through the PR in some neurons.
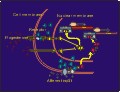 Click to enlarge
Figure 6: Neuron containing PRs and in which afferent input from VCS activates PRs, which may then influence expression of progestin responsive genes, resulting in changes in expression of specific mRNA's and proteins.
Click to enlarge
Figure 6: Neuron containing PRs and in which afferent input from VCS activates PRs, which may then influence expression of progestin responsive genes, resulting in changes in expression of specific mRNA's and proteins.
Furthermore, other work (discussed in symposium entitled: Genital sensation: CNS targets and functions in females) suggests that stimuli from the social environment may directly influence activation and regulation of the concentration of PRs in these neurons, causing PR-dependent gene expression and changes in behavioral response to progesterone (Figure 6).
References
- Auger, AP and Blaustein, JE (1995) Progesterone enhances an estradiol-induced increase in Fos immunoreactivity in localized regions of female rat forebrain. Journal of Neuroscience, 15: 2272 - 2279.
- Auger, AP and Blaustein, JD (1997) Progesterone treatment increases Fos-immunoreactivity within some progestin receptor-containing neurons in localized regions of female rat forebrain. Brain Research, 746: 164 - 170.
- Auger, AP, Moffatt, CA and Blaustein, JD (1996) Reproductively-relevant stimuli induce Fos-immunoreactivity within progestin receptor-containing neurons in localized regions of female rat forebrain. Endocrinology, 138: 511 - 514.
- Auger, AP, Moffatt, CA and Blaustein, JD (1997) Progesterone-independent activation of rat brain progestin receptors by reproductive stimuli. Endocrinology, 138: 511 - 514.
- Blaustein, JD and Olster, DH (1989) Gonadal steroid receptors and social behavior. In Advances in Comparative and Environmental Physiology, (J Balthazart, Ed.), pp 31 - 104, Springer Verlag, Berlin.
- Mani, SK, Blaustein, JD and O'Malley, BW. (1997) Progesterone receptor function from a behavioral perspective. Hormones and Behavior, 31: 244 - 255.
- Meredith, JM, Auger, AP and Blaustein, JD (1997) D1 dopamine receptor agonist (SKF-38393) induction of fos immunoreactivity in progestin receptor-containing areas of female rat brain. Journal of Neuroendocrinology, 9: 385 - 394.
- Tetel, MJ, Getzinger, MJ and Blaustein, JD (1993) Fos expression in the rat brain following vaginal-cervical stimulation by mating and manual probing. Journal of Neuroendocrinology, 5: 397 - 404.
- Tetel, MJ, Celentano, DC and Blaustein, JD (1994) Intraneuronal convergence of tactile and hormonal stimuli associated with female reproduction in rats. Journal of Neuroendocrinology, 6: 211 - 216.
| Discussion Board | Previous Page | Your Symposium |