Invited Symposium: Cytokines, Monoamines and Behavior
| INABIS '98 Home Page | Your Session | Symposia & Poster Sessions | Plenary Sessions | Exhibitors' Foyer | Personal Itinerary | New Search |
Introduction
Autoimmunity-Associated Behavioral Syndrome in MRL-lpr mice The onset of chronic autoimmune, lupus-like disease in MRL/MpJ-lpr (MRL-lpr) mice is characterized by an imbalance in the cytokine network, autoantibody hyperproduction and a constellation of behavioral deficits, labelled “autoimmunity-associated behavioral syndrome” or AABS (1). As observed in many patients suffering from the systemic lupus erythematosus (SLE)(2; 3), the behavioral deficits at the onset of autoimmunity suggest a progressive anxious/depressive-like behavioral state. When compared to age-matched MRL/MpJ-+/+ (MRL +/+) controls (which develop a late form of the disease), MRL-lpr mice show increased thigmotaxic behavior (4; 5),impaired exploration of novel objects and spaces, excessive floating in the forced swim test (6), reduced intake of sucrose solution and water (7), response perseveration in a spatial learning task(4), and reduced isolation-induced intermale fighting (8). The assumption that early serologic changes induce AABS is based on the evidence that immunosuppressive treatment concurrently reduces immunological and behavioral differences between the MRL-lpr and the control MRL +/+ substrain (7; 9) and that the severity of the behavioral impairments correlate with serum autoantibody (6; 10) and interleukin-6 (IL-6) levels in MRL-lpr mice (11). In addition, most of the behavioral deficits appear well before an age when severe peripheral symptomatolgy may occur, and thus could not be accounted by impaired motor performance (4; 12; 13).
Neuropathological changes in MRL-lpr mice are proposed to represent, at least in part, a structural basis for some aspects of AABS. They include ventricular enlargement (14), lymphoid cell infiltration into the chorioid plexus and brain parenchyma (15; 16), meningitis (17), reduced brain weight and marked morphological changes in the hippocampus and parietal cortex (18). In particular, using Golgi-impregnation and Cresyl-violet staining methods, we revealed a progressive neurodegeneration, which was characterized by reduced dendritic branching and spine density (Figure 1), as well as reduced cell size and density of pyramidal neurons in the parietal cortex and in the CA1 region of the hippocampus. Importantly, the degree of morphological changes correlated with the severity of autoimmune disease. The hypothesis that dendritic dysmorphology is induced by developing autoimmunity and inflammation was recently supported by the evidence that prolonged immunosuppressive treatment with cyclophosphamide prevents changes in dendritic branching (19).
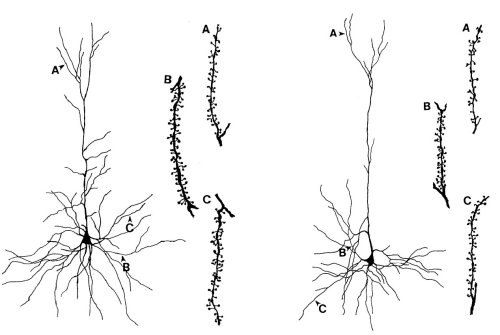 Figure 1. Reduced dendritic branching and spine density in pyramidal neurons of the parietal cortex from autoimmune MRL-lpr mice. Apical (A), basilar medial (B) and basilar terminal dendritic segments (C) are shown with a typical spine distribution.
Figure 1. Reduced dendritic branching and spine density in pyramidal neurons of the parietal cortex from autoimmune MRL-lpr mice. Apical (A), basilar medial (B) and basilar terminal dendritic segments (C) are shown with a typical spine distribution.
Proposed immunopathogenic factors Although ample evidence suggests a pathogenic role for brain-reactive antibodies in the etiology of aberrant behavior (20; 21), it is evident that many deficits cannot be accounted for solely on the basis of this factor. Psychiatric symptoms may occur in SLE patients even before the appearance of autoantibodies (3) and only some of the variety of behavioral deficits in lupus-prone mice are associated with the presence of autoantibodies (6; 10). Imbalance in cytokine secretion is another hallmark of autoimmunity (22) and emerging evidence indicates that cytokines are potent modulators of behavior, both in health and disease (23). In addition to their regulatory role in the immune and the endocrine systems (24), pro-inflammatory cytokines IL-1, IL-6, and TNF-alpha and their cognate receptors in the brain (25) regulate neural plasticity, temperature control and sleep (26) and the HPA axis activity (23; 24; 27-30).
Interleukin-6 (IL-6) The rationale for focussing on IL-6 as one of a key factors in etiology of AABS and neurodegeneration is based on the following evidence. First, of all the neuroactive cytokines known to be altered in MRL-lpr mice (31-33) IL-6 is the first one to be overexpressed in an age-dependant manner in the serum (34). Second, aberrant behavior appears before autoantibody hyperproduction is detected (11) and coincides temporally with IL-6 overexpression (34). Third, blunted responsiveness to sucrose correlates with high levels of serum IL-6, and is not seen in MRL-lpr mice in which IL-6 activity is abolished by immunosuppressive treatment (11) (Figure 2).
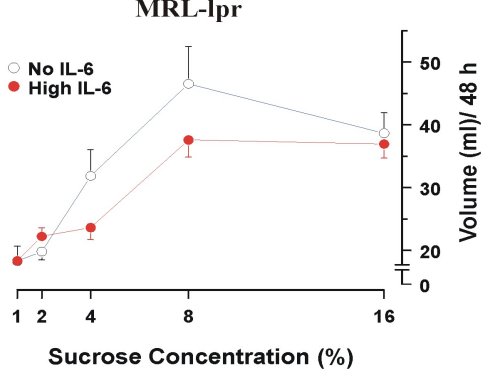 Figure 2. Increased responsiveness to sucrose solutions in IL-6-free MRL-lpr mice after the treatment of immunosuppresice drug cyclophosphamide (100 mg/kg/week).
Figure 2. Increased responsiveness to sucrose solutions in IL-6-free MRL-lpr mice after the treatment of immunosuppresice drug cyclophosphamide (100 mg/kg/week).
Fourth, reduced preference for sucrose can be induced in healthy mice by an adeno-virus carrying murine IL-6 cDNA, but not by the same virus without the IL-6 gene (11) (Figure 3).
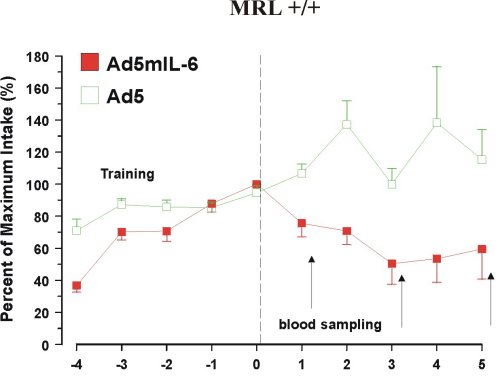 Figure 3. Reduced intake of 4% sucrose over 5 days in mice infected with Ad5mIL-6 adenovirus.
Figure 3. Reduced intake of 4% sucrose over 5 days in mice infected with Ad5mIL-6 adenovirus.
Finally, although we could not detect IL-6 mRNA by in situ hybridization or immunohistochemistry (unpublished data), Tsai and colleagues have observed IL-6 mRNA in the brains of MRL-lpr mice with RT-PCR methodology (31). Along the same line, autoimmune MRL-lpr mice (18; 35; 36) show comparable pathology to transgenic mice which chronically overexpress IL-6, as evidenced by aberrant dendritic morphology in the CA1 region of the hippocampus (37) and elevated plasma corticosterone (CORT) levels (38).
Materials and Methods
Infection with IL-6 Adenovirus Vector (Ad5mIL6) To examine behavioral effects of prolonged exposure to IL-6 and circumvent the problem of the short serum half-life of IL-6 (39), healthy MRL +/+ and AKR/J mice were infected with an adenovirus vector carrying the gene for murine IL-6 (Ad5mIL6). A lupus-prone but asymptomatic MRL +/+ mice and healthy ancestral AKR/J strain were selected to examine how general are the effects of adenoviral infections. The Ad5mIL-6 virus is a recombinant human type 5 adenovirus constructed to carry murine IL-6 cDNA in the E3 region of the genome (40). When injected intraperitoneally, Ad5mIL-6 produces a significant increase of biologically active IL-6, likely due to infection of hepatocytes, splenocytes and peritoneal cells. Elevated serum IL-6 levels can be observed up to 5-6 days post-infection (11) (Figure 4). The adenovirus devoid of the IL-6 gene in the E3 region of the genome (Ad5) was used as a control type of virus. Ad5 does not produce IL-6 upon infection of cells in culture, nor it causes a significant elevation of serum IL-6 levels in mice when injected i.p. (40).
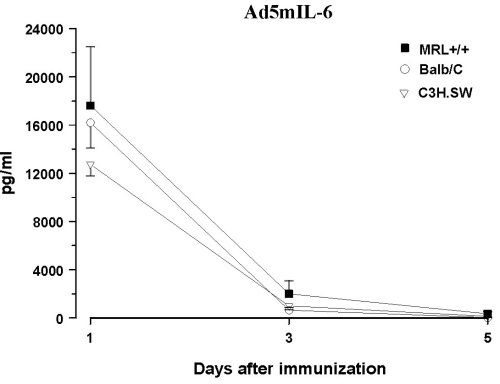
Figure 4. Serum levels of bioactive IL-6 in three strains of mice infected with AD5mIL-6 or control, wild-type AD5 adenovirus. Note the difference in Y-axis scale.
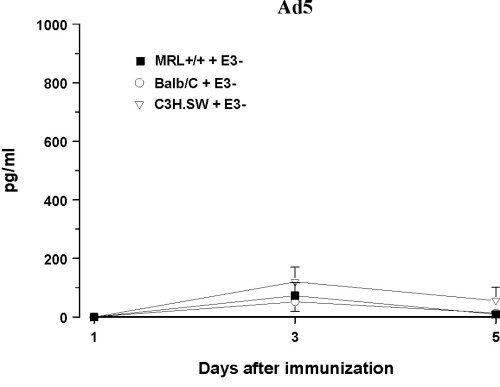
The baseline performance was estimated in several behavioral tasks given over 5 days. When infected, half of the mice from each strain were infected with either Ad5mIL-6 or control wild-type Ad5 adenovirus (i.p. 2x108 pfu of virus/mouse).
Results
Behavioral effects of Ad5mIL-6 infection were examined in the forced swim (Porsolt’s) test, the novel object test and the plus maze test, but no consistent changes in the performance were found across the strains. Reduced intake of sucrose was replicated in both strains of mice infected with the Ad5mIL-6 virus (data not shown). However, reduced food (Figure 5) and water intake (Figure 6), as well as reduced body weight (data not presented) had accompanied this effect, suggesting an overall impairment in ingestive behavior after prolonged exposure to IL-6. A significant drop in rectal temperature was observed in AKR/J mice several days after infection (Figure 7). Given that behavioral effects were transient, the brains from infected mice were not processed for the Golgi or Cresyl-violet stainings.
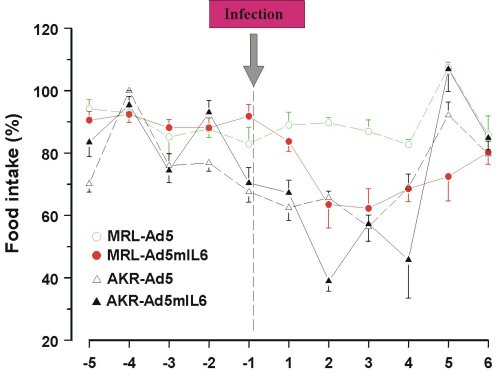 Figure 5. Reduced food intake over 6 days in mice infected with Ad5mIL-6 adenovirus.
Figure 5. Reduced food intake over 6 days in mice infected with Ad5mIL-6 adenovirus.
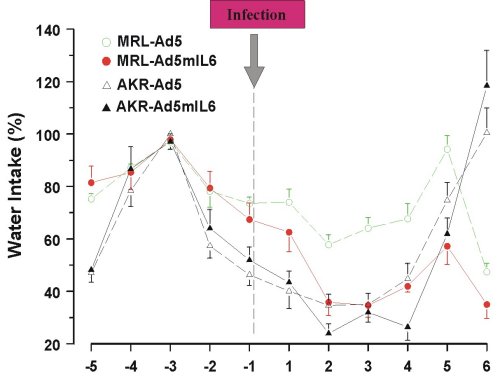 Figure 6. Reduced water intake over 6 days in mice infected with Ad5mIL-6 adenovirus.
Figure 6. Reduced water intake over 6 days in mice infected with Ad5mIL-6 adenovirus.
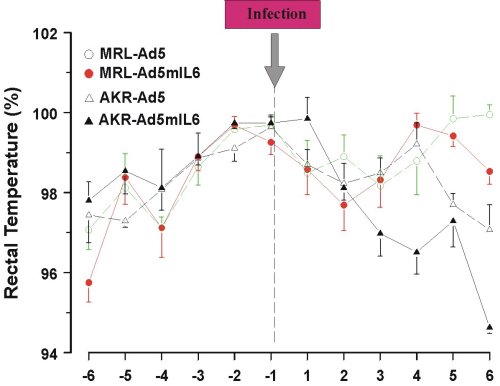 Figure 7. Reduced rectal temperature in AKR/J mice infected with Ad5mIL-6 adenovirus
Figure 7. Reduced rectal temperature in AKR/J mice infected with Ad5mIL-6 adenovirus
Discussion and Conclusion
The present findings suggest that prolonged exposure to circulating IL-6 transiently impairs ingestive (cosummatory) behavior, as evidenced by reduced sucrose, food and water intake over 6 days in mice infected with Ad5mIL-6 adenovirus. There were no other significant or general changes in locomotor activity, exploratory behavior or performance in the forced swim test.
These results are in accordance with the lack of significant effects in the spatial learning task (41) or the plus-maze (42; 43) after acute central (i.c.v.) administration of IL-6. Our results with Ad5mIL-6 adenovirus are also consistent with the evidence that secretion of IL-6 from the developed tumors is associated with decreased food consumption, reduced body weight, and reduced blood glucose levels (44). It is possible that the present results are rather related to known IL-6 effects on hepatic function, as evidenced by changes in plasma glucagon, glucose and a depletion of hepatic glycogen (45). If chronic IL-6 indeed lowers metabolic requirements and reduces cerebral regional blood volume (46), it is possible that chronic IL-6-induced hypoglycemia affects neuronal growth and survival in autoimmune MRL-lpr mice. This is supported by recent repeated measurement of glucose levels which indicates an age-dependent decrease in glucose levels in MRL-lpr mice (unpublished results). However, this hypothesis needs to be further examined by employing other endocrine, neuromorphological and physiological measures in animals chronically exposed to IL-6.
References
1. Sakic B, Szechtman H, Denburg JA. Neurobehavioral alteration in autoimmune mice. Neurosci.Biobehav.Rev. 1997; 21: 327-340.
2. Wekking EM. Psychiatric symptoms in systemic lupus erythematosus - an update. Psychosom.Med. 1993; 55: 219-228.
3. van Dam AP, Wekking EM, Callewaert JAC, et al. Psychiatric symptoms before systemic lupus erythematosus is diagnosed. Rheumatol.Int. 1994; 14: 57-62.
4. Sakic B, Szechtman H, Keffer M, Talangbayan H, Stead R, Denburg JA. A behavioral profile of autoimmune lupus-prone MRL mice. Brain Behav.Immun. 1992; 6: 265-285.
5. Sakic B, Szechtman H, Denburg SD, Carbotte RM, Denburg JA. Spatial learning during the course of autoimmune disease in MRL mice. Behav.Brain Res. 1993; 54: 57-66.
6. Sakic B, Szechtman H, Talangbayan H, Denburg SD, Carbotte RM, Denburg JA. Disturbed emotionality in autoimmune MRL-lpr mice. Physiol.Behav. 1994; 56: 609-617.
7. Sakic B, Denburg JA, Denburg SD, Szechtman H. Blunted sensitivity to sucrose in autoimmune MRL-lpr mice: a curve-shift study. Brain Res.Bull. 1996; 41: 305-311.
8. Sakic B, Gurunlian L, Denburg SD. Reduced aggressiveness and low testosterone levels in autoimmune MRL-lpr males. Physiol.Behav. 1998; 63: 305-309.
9. Sakic B, Szechtman H, Denburg SD, Denburg JA. Immunosuppressive treatment prevents behavioral deficit in autoimmune MRL-lpr mice. Physiol.Behav. 1995; 58: 797-802.
10. Sakic B, Szechtman H, Denburg SD, Carbotte RM, Denburg JA. Brain-reactive antibodies and behavior of autoimmune MRL-lpr mice. Physiol.Behav. 1993; 54: 1025-1029.
11. Sakic B, Szechtman H, Braciak TA, Richards CD, Gauldie J, Denburg JA. Reduced preference for sucrose in autoimmune mice: a possible role of interleukin-6. Brain Res.Bull. 1997; 44: 155-165.
12. Sakic B, Szechtman H, Stead R, Denburg JA. Joint pathology and behavioral performance in autoimmune MRL-lpr mice. Physiol.Behav. 1996; 60: 901-905.
13. Brey RL, Cote S, Barohn R, Jackson C, Crawley R, Teale JM. Model for the neuromuscular complications of systemic lupus erythematosus. Lupus 1995; 4: 209-212.
14. Denenberg VH, Sherman GF, Rosen GD, Morrison L, Behan PO, Galaburda AM. A behavior profile of the MRL/Mp lpr/lpr mouse and its association with hydrocephalus. Brain Behav.Immun. 1992; 6: 40-49.
15. Vogelweid CM, Johnson GC, Besch-Williford CL, Basler J, Walker SE. Inflammatory central nervous system disease in lupus-prone MRL/lpr mice: comparative histologic and immunohistochemical findings. J.Neuroimmunol. 1991; 35: 89-99.
16. Farrell M, Sakic B, Szechtman H, Denburg JA. Effect of cyclophosphamide on leucocytic infiltration in the brain of MRL/lpr mice. Lupus 1997; 6: 268-274.
17. Alexander EL, Murphy ED, Roths JB, Alexander GE. Congenic autoimmune murine models of central nervous system disease in connective tissue disorders. Ann.Neurol. 1983; 14: 242-248.
18. Sakic B, Szechtman H, Denburg JA, Gorny G, Kolb B, Whishaw IQ. Progressive atrophy of pyramidal neuron dendrites in autoimmune MRL-lpr mice. J.Neuroimmunol. 1998; 87: 162-170.
19. Sakic B, Whishaw IQ, Kolb B, Denburg JA, Szechtman H. Impaired spontaneous alternation behavior in autoimmune MRL-lpr mice: relationship to hippocampal damage and cyclophosphamide treatment. Neuroimmunomodulation. 1998; 5: 103(Abstract)
20. Denburg JA, Denburg SD, Carbotte RM, Sakic B, Szechtman H. Nervous system lupus: pathogenesis and rationale for therapy. Scan.J.Rheumatol. 1995; 12: 263-273.
21. Hoffman SA, Narendran A, Shucard DW, Harbeck RJ. Autoantibodies, immune complexes, and behavioral disorders: Neuropsychiatric involvement in systemic lupus erythematosus. Drug Dev.Res. 1988; 15: 237-251.
22. Crow MK, Christian CL. Etiologic hypotheses for systemic lupus erythematosus. In: Lahita RG, ed. Systemic Lupus Erythematosus. New York: Churchill Livingstone, 1992: 51-64.
23.Plata-Salaman CR. Immunoregulators in the nervous system. Neurosci.Biobehav.Rev. 1991; 15: 185-215.
24.Imura H, Fukata J, Mori T. Cytokines and endocrine function: an interaction between the immune and neuroendocrine systems. Clin.Endocrinol. 1991; 35: 107-115.
25. Schobitz B, De Kloet ER, Holsboer F. Gene expression and function of interleukin 1, interleukin 6 and tumor necrosis factor in the brain. Prog.Neurobiol. 1994; 44: 397-432.
26. Dantzer R, Bluthe RM, Kent S, Kelley KW. Cytokines and sickness behavior. In: Husband AJ, ed. Psychoimmunology: CNS-Immune Interactions. Boca Raton: CRC Press, Inc., 1994: 1-16.
27.Turnbull AV, Rivier C. Regulation of the HPA axis by cytokines. Brain Behav.Immun. 1995; 9: 253-275.
28. Sternberg EM, Chrousos GP, Wilder RL, Gold PW. The stress response and the regulation of inflammatory disease. Ann.Int.Med. 1992; 117: 854-866.
29. Sweep F, Rijnkels C, Hermus A. Activation of the hypothalamus-pituitary-adrenal axis by cytokines. Acta Endocrinol. 1991; 125 Suppl 1: 84-91.
30. Rivier C, Rivest S. Mechanisms mediating the effects of cytokines on neuroendocrine functions in the rat. In: Chadwick D, Marsh JAckrill K, eds. Corticotropin-Releasing Factor. Chichester: John Wiley & Sons, 1993: 204-225.
31. Tsai CY, Wu TH, Huang SF, et al. Abnormal splenic and thymic IL-4 and TNF-alpha expression in MRL-lpr/lpr mice. Scan.J.Immunol. 1995; 41: 157-163.
32. Levine JS, Pugh BJ, Hartwell D, Fitzpatrick JM, Marshak-Rothstein A. Interleukin-1 dysregulation is an intrinsic defect in macrophages from MRL autoimmune-prone mice. Eur.J.Immunol. 1993; 23: 2951-2958.
33. Magilavy DB, Rothstein JL. Spontaneous production of tumor necrosis factor alpha by Kupffer cells of MRL/lpr mice. J.Exp.Med. 1988; 168: 789-794.
34. Tang B, Matsuda T, Akira S, et al. Age-associated increase in interleukin 6 in MRL/lpr mice. Int.Immunol. 1991; 3: 273-278.
35. Hu Y, Dietrich H, Herold M, Heinrich PC, Wick G. Disturbed immuno-endocrine communication via the hypothalamo- pituitary-adrenal axis in autoimmune disease. Int.Arch.Allergy Immunol. 1993; 102: 232-24136.
36. Lechner O, Hu Y, Jafarian-Tehrani M, et al. Disturbed immunoendocrine communication via the hypothalamo- pituitary-adrenal axis in murine lupus. Brain Behav.Immun. 1996; 10: 337-350.
37. Campbell IL, Abraham CR, Masliah E, et al. Neurologic disease induced in transgenic mice by cerebral overexpression of interleukin 6. Proc.Natl.Acad.Sci.U.S.A. 1993; 90: 10061-10065.
38. Raber J, O'Shea RD, Bloom FE, Campbell IL. Modulation of hypothalamic-pituitary-adrenal function by transgenic expression of interleukin-6 in the CNS of mice. J.Neurosci. 1997; 17: 9473-9480.
39. Castell JV, Geiger T, Gross V, et al. Plasma clearance, organ distribution and target cells of interleukin-6/hepatocyte-stimulating factor in the rat. Eur.J.Biochem. 1988; 177: 357-361.
40. Braciak TA, Mittal SK, Graham FL, Richards CD, Gauldie J. Construction of recombinant human type 5 adenoviruses expressing rodent IL-6 genes. An approach to investigate in vivo cytokine function. J.Immunol. 1993; 151: 5145-5153.
41. Oitzl MS, van Oers H, Schobitz B, De Kloet ER. Interleukin-1 beta, but not interleukin-6, impairs spatial navigation learning. Brain Res. 1993; 613: 160-163.
42. Connor TJ, Song C, Leonard BE, Merali Z, Anisman H. An assessment of the effects of central interleukin-1 beta, -2, -6, and tumor necrosis factor-alpha administration on some behavioural, neurochemical, endocrine and immune parameters in the rat. Neuroscience 1998; 84: 923-933.
43. Song C, Connor TJ, Anisman H, Ravindran AV, Merali Z. Comparative effect of acute central cytokine administration on behavioral, neurochemical, endocrine and immune parameters in the rat. Soc.Neurosci.Abst. 1996; 22: 847(Abstract)
44. Metzger S, Goldschmidt N, Barash V, et al. Interleukin-6 secretion in mice is associated with reduced glucose-6- phosphatase and liver glycogen levels. Am.J.Physiol. 1997; 273: E262-E267
45. Stith RD, Luo J. Endocrine and carbohydrate responses to interleukin-6 in vivo. Circul.Shock 1994; 44: 210-215.
46. Saija A, Princi P, Lanza M, Scalese M, Aramnejad E, De Sarro A. Systemic cytokine administration can affect blood-brain barrier permeability in the rat. Life Sci. 1995; 56: 775-784.
| Discussion Board | Previous Page | Your Symposium |