| INABIS '98 Home Page | Your Poster Session | Related Symposia & Posters | Plenary Sessions | Exhibitors' Foyer | Personal Itinerary | New Search |
Poster Number: PAmatsuoka0124
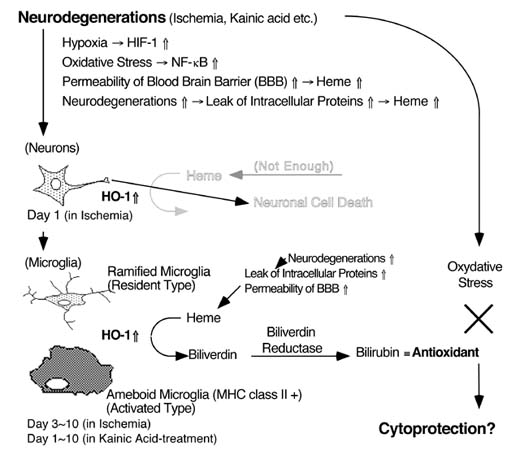
The molecular and cellular events responsible for the selective vulnerability of the population of neurons to ischemia and kainate-induced seizure activity are not yet understood. However, oxidative stress possibly participated in these neurodegenerations. Heme oxygenase (HO) is oxidation of the heme in concert with P-450 reductase followed by the specific cleavage of heme into biliverdin, CO, and iron. The biliverdin, is rapidly metabolized by biliverdin reductase to bilirubin, which is a powerful antioxidant. Therefore HO expression may showed the selfprotective effects. In this study, we examined the changes of HO in ischemia- and kainate-induced neurodegenerations. Although inducible HO (HO-1) is expressed limited number of the neurons in the control, HO-1 was strongly induced by ischemia and kainate-injection. HO-1 was expressed in the pyramidal neurons 1 day after ischemia, and in the microglia 3-7 days after ischemia and 1-7 days after kainate-injection. HO-1 expression in the microglia was colocalized with major histocompatibility complex (MHC) class II antigen. These results indicate that: i) HO-1 was strongly induced by oxidative stress; ii) HO-1 expression possibly participated in oxidative stress-induced neurodegeneration; and iii) ameboid microglia, which express both HO-1 and MHC antigens, may play a key role in neurodegeneration induced by ischemia and kainate.
Keywords: heme oxygenase, ischemia, kainate, major histocompatibility complex
One function of heme oxygenase (HO) is oxidation of the heme molecule in concert with NADPH-cytochrome P450 reductase and then specific cleavage of heme into biliverdin, carbon monoxide (CO), and iron. Previous studies have indicated that there are at least two isozymes of HO, i.e., an inducible type (HO-1) and a constitutive type (HO-2). One metabolite of heme, biliverdin, is rapidly metabolized by biliverdin reductase to bilirubin, which has potent antioxidant activities. In addition, HO is the rate limiting enzyme in the formation of bilirubin. Induction of HO-1 may protect cells from oxidative stress by increasing the formation of bilirubin, which has antioxidant properties, and by fostering the sequestration of catalytic iron with ferritin.
Major histocompatibility complex (MHC) antigens are well known as glycoproteins of cell surface that play central roles in self-recognition in the immune system. Previously, the CNS is considered to be immunologically privileged. However, recent evidences suggest that MHC antigens are induced in experimental animal models by brain inflammations or neurotoxins, and in human neurological disorders such as Alzheimerユs diseases. These observations suggest that MHC antigens also play critical roles in a degeneration of the brain.
The molecular and cellular basis of the neurodegeneration induced by kainic acid (KA) and ischemia remains unclear. However, excessive release of glutamate and oxidative stress may participates. Therefore, we tried to clarify whether or not HO-1 is induced by KA and ischemia.
-Materials:
anti-rat HO-1 antibody
StressGen (Victoria, Canada)
anti-rat HO-2 antibody
StressGen (Victoria, Canada)
anti-GFAP antibody
Chemicon International (Temecula, CA)
anti-rat CD11b antibody
Serotec Ltd. (Oxford, U.K.)
anti-rat MHC class I antibody
Serotec Ltd. (Oxford, U.K.)
anti-rat MHC class II antibody
Serotec Ltd. (Oxford, U.K.)
Bradford protein assay kit
BioRad Laboratories (Hercules, CA)
Vectastain ABC Elite kit
Vector Laboratories (Burlingname, CA
Enhanced chemiluminescence detection system
Amersham (Buckinghamshire, U.K.)
-Animal Models (Kainic Acid & Transient Forebrain Ischemia):
Male Wistar rats (Charles River, Japan) were used. The experimental protocol was approved by the Committee for Animal Research at Kyoto Pharmaceutical University. Rats were fasted over night with free access to water.
KA (1 ug KA in 2 uL of sterilized physiological saline) was injected right lateral ventricle under anesthetics.
Transient forebrain ischemia (50 mmHg for 8 minutes) was treated as previously reported (Smith et al. (1984) Acta. Neuropathol. (Berl) 64: 319-332) with slight modifications (Kaku et al. (1993) J. Cereb. Blood Flow Metab. 13: 402-408).
-Cell Culture
Glial cells (mixture of astrocytes and microglia) were prepraed as established method. Glial cells were ated with KA for 24 hours, and then analyzed.
-Samples were analyzed by using immunoblotting and immunohistochemistry. (see these papers for details).
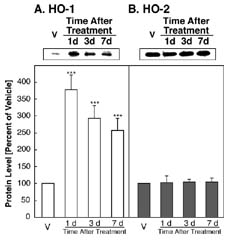
FIG. 1: Alterations of HO-1 and HO-2 in the cytosolic fraction of the rat hippocampus after i.c.v. injection with vehicle (V), or 1 ug KA. [View larger version in a new window (102K)]
HO-1 levels were significantly enhanced 1, 3 and 7 days after i.c.v. injection of KA (Fig. 1A). One day after i.c.v. injection of KA, the HO-1 protein level reached a maximum and then decreased, but was still significantly enhanced versus the vehicle-injected group (Fig. 1A). On the other hand, in the rat hippocampus, as shown in Fig. 1B, the protein band for HO-2 is stronger than that of HO-1. The 36-kDa HO-2 protein level did not change after i.c.v. injection of the vehicle or KA (Fig 1B). Quantitatively, the 33-kDa HO-1 protein was significantly enhanced after i.c.v. injection of KA, with increases of 378% (P<0.01), 292% (P<0.01) and 258% (P<0.01) at 1, 3, and 7 days, respectively, relative to injection of the vehicle (Fig. 1A).
FIG. 2: HO-1-immunoreactivity in rat hippocampus. [View larger version in a new window (349K)]
In rats received vehicle injection, HO-1-immunoreactivity was observed in a limited area, i.e., strongly in some of neurons in the hilus of the dentate gyrus (Fig. 2A), while hard to detect in the other subfields and in glial cells (Fig. 2A). After i.c.v. injection of KA, HO-1-immunoreactivity was strongly induced not only in the CA3 but also widely in the whole hippocampus (Fig. 2B).
FIG. 3: Photomicrographs of immunostaining using antibodies against CD11b, GFAP, and HO-1, 1 day after i.c.v. injection with vehicle or 1 ug of KA. [View larger version in a new window (519K)]
In the hippocampus from non-injected and vehicle-injected rats, CD11b-immunoreactive microglia have thin and longer process that is typical ramified type (Fig. 3A). After KA-injection, numerous CD11b-immunoreactive microglia which have thick and shorter process, i.e., typical ameboid type, in the CA3 subfield (Fig. 3B). In the other hippocampal subfields, however, microglia is mostly ramified type. GFAP-immunoreactive astrocytes were slightly activated by injection with KA (Figs. 3C and 3D). Although HO-1-immunoreactivity was not detected in vehicle-injected in the CA3 subfield (Fig. 3E), after KA injection, HO-1-immunoreactivity was strongly induced in the cells which are morphologically similar to the glial cells (Fig. 3F). HO-1 immunoreactivity was detected 6 h after injection with KA, and the maximal immunoreactivity was observed after 1 day. Subsequently, HO-1 immunoreactivity was gradually decreased after 3 to 7 days, and retured to control level after approximately 10 days (data not shown).
FIG. 4: Double immunostaining with HO-1 and glial markers, CD11b for microglia and GFAP for astrocytes, and MHC class I and class II. [View larger version in a new window (162K)]
To clarify the cell types that express HO-1 protein, we performed double immunostaining with HO-1 and glial cell markers, i.e., anti-CD11b antibody for microglia and anti-GFAP antibody for astrocytes. Double immunolabeling indicates that HO-1-immunoreactive cells were observed predominantly in the CD11b-immunoreactive microglia (Figs. 4A and 4C), and also in the GFAP-immunoreactive astrocytes (Figs. 4B and 4D). Double immunostaining indicates that HO-1-immunoreactivity was predominantly colocalized with MHC class II-immunoreactivity (Fig. 4F), and slightly colocalized with MHC class I-immunoreactivity (Fig. 4E).
FIG. 5:
Photomicrographs of immunostaining using antibodies against CD11b,
GFAP, and HO-1 in cultured rat glial cells in vitro.
The mixed glial cells were treated with vehicle or 1 mM KA for 24 h. [View larger version in a new window (298)]
In the mixed glial cells used this study, approximately 7% of cells were CD11b-immunoreactive microglia, and over 90% were GFAP-immunoreactive astrocytes. In comparison with CD11b- and GFAP-immunoreactivities, HO-1 immunoreactivity was observed predominantly in microglia but not in astrocytes in the vehicle-treatment (Fig. 5E). In contrast, treatment with 1 mM KA for 24 h markedly induced HO-1-immunoreactivity in the astrocytes (Fig. 5F). Thus, HO-1-immunoreactivity in KA-treated glial cells are observed in both microglia and astrocytes in cultured cells.
FIG. 6:
Alterations of proteins of HO-1 and HO-2 after ischemia in the
cytosolic fraction from the rat hippocampus. [View
larger version in a new window (94K)]
Although protein levels of HO-1 did not change with the sham operation, HO-1 expression levels were markedly enhanced on days 1, 3, and 7 after ischemia (Fig. 6A). Conversely, the level of the 36-kDa HO-2 protein was detected in higher levels than the HO-1 protein in the untreated hippocampus, and the expression level of HO-2 was not altered by the sham operation or by ischemia (Fig. 6B). Quantitatively, the level of the HO-1 protein increased significantly on day 1, 227% (P<0.05), day 3, 277% (P<0.05), and day 7, 330% (P<0.01) after ischemia (Fig. 6A), whereas the level of the HO-2 protein was unchanged (Fig. 6B).
FIG. 7.
HO-1-immunoreactivity in rat hippocampal formation after
sham-operation or ischemia.
[View larger version in a new window
(221K)]
See bellow.
FIG. 8. HO-1
immunoreactivity in the CA1 subfield after ischemia. [View
larger version in a new window (340K)]
HO-1 immunoreactivity was induced in the CA1 subfield, but was not induced in other subfields after ischemia (Figs. 7B and 7C). On day 1 after ischemia, HO-1 immunoreactivity was observed in the pyramidal neurons in the CA1 subfield (Figs. 7B, 8B, and 8E). Subsequently, on days 3 and 7 after ischemia, HO-1 immunoreactivity was observed in the glial-like cells, but not in neurons in the CA1 subfield (Figs. 7C, 8C, 8D, and 8F).
FIG. 9. Double
immunostaining with HO-1 and glial cell markers, CD11b for microglia
and GFAP for astrocytes, and MHC class I and class II.
HO-1-immunoreactivity was detected by blue staining and other immunoreactivity was detected by brown staining. Double positive cells are indicated by closed arrow heads, and single positive cells are indicated by open arrow heads. [View larger version in a new window (255K)]
After ischemia, MHC immunoreactivities were induced in glial cells in the CA1 subfield. After 3 days, the immunoreactivities of MHC class I and II in ameboid microglia were observed in the CA1 subfield, but not in the other subfields in the hippocampal formation (data not shown). In addition, HO-1 immunoreactivity in ameboid microglia was mainly co-localized with MHC class II (Fig. 9F) and little with class I (Fig. 9E).
In the rat hippocampal formation in vivo, constitutive expression of HO-1 mRNA and its protein are at a very low level and HO-1-immunoreactivity is observed in a limited number of neurons. On the other hand, HO-2 is much more widely expressed in the neurons. Recently, it was suggested that HO also functions as a defense system against oxidative stress, since biliverdin and bilirubin produced locally may act as physiological antioxidants and potent scavengers of oxygen radicals. Thus, the biliverdin and bilirubin produced by HO are important antioxidants in stressed cells. The molecular and cellular basis of the neurodegenerative mechanism remains unclear, however, the excess release of glutamates and oxidative stress may be participate.
<Conclusion> <References> <Discussion Board>
Although HO-1 was significantly enhanced, HO-2 was not changed in KA-induced episode. HO-1 and biliverdin reductase, but not HO-2, were resistant to heat shock. In addition, HO-2 activity in the brain was inhibited by nitric oxide (NO). In contrast to rat and human HO-2 proteins which contain three and two cysteine residues, respectively, rat and human HO-1 proteins have no cysteine residue. Since NO induced S-nitrosylation and ADP-ribosylation of cysteine residues in several enzymes and resulted in reduction of its enzyme activity, HO-1 protein may not be influenced by NO. These observations suggest that HO-1 is more effective rather than HO-2 under brain injury. Recently, it was suggested that HO also functions as a defense system against oxidative stress, because biliverdin and bilirubin produced locally may work as physiological antioxidants and potent scavengers of oxygen radicals. In fact, overexpression of HO-1 exhibited marked decreases in cell growth and DNA synthesis, and increased survival to hyperoxic oxidant injury. From these observations, we consider that numerous glial cells in which HO-1 protein was induced may be resistant to oxidative stress such as NO. On the other hand, for the production of biliverdin, bilirubin and CO by HO-1 is necessary to a substrate heme. With respect to a source of heme, we considered two possibilities. First, since the permeability of blood brain barrier was significantly upregulated, heme may be supplied early from blood proteins. Another, delayed release of heme may be from dead neurons by stimuli.
<Conclusion> <References> <Discussion Board>
In the normal and vehicle-injected rat hippocampi, neither MHC class I nor class II was detectable. Both MHC class I and II were expressed in ameboid microglia, but not in astrocytes, surrounding the area in which neuronal damage was occurring. Several recent papers described KA neurotoxicity and ischemia induced in MHC class II-immunoreactive ameboid microglia. In addition, reactive microglia that express MHC class II have been observed phagocytosing degenerated neuronal elements in Alzheimer's disease, Parkinson's disease, acquired immunodeficiency syndrome (AIDS), and some other neuronal degenerative disorders. In this study, we showed that HO-1-immunoreactive cells were colocalized with both MHC class I- and II-immunoreactive ameboid microglia, and the induction of HO-1 preceded induction of MHC antigens. These observations suggest that microglia which induces HO-1 protein acquires resistance to oxidative stress and then expresses MHC class II and/or I. Therefore, we consider that microglia which express both HO-1 and MHC antigens may play a repair of degenerative area and a reconstruction the neuronal functions and/or a scavenging of dead neurons, in delayed episode of ischemia and KA-injection.
<Conclusion> <References> <Discussion Board>
In conclusion, we examined the induction of HO-1 and MHC antigens in the rat brain after KA-treatment and ischemia. The big question that "Is HO-1 harmful or helpful?" is still unclear. However, we investigate that HO-1 was predominantly expressed MHC class II-expressing microglial cells. In contrast to the neurons, HO-1 expressing microglial cells were activated after neurodegeneration. These results suggest that MHC class II-expressing microglial cells may play critical roles in neurodegenerative episode. On the other hand, HO-1 was transiently expressed in the CA1 pyramidal neurons after transient forebrain ischemia. This result looks ambiguous since HO-1-expressing CA1 pyramidal neurons undergoing apoptosis. Detail analysis of the relationship between HO-1 and neurons which undergoing apoptosis is necessary before definitive conclusions can be drawn.
Conclusion: HO-1 functions after neurodegeneration in the CNS.
Acknoledgements: Y.M. is the recipient of a Research Fellowship from the Japan Society for the Promotion of Science. This paper was supported in part by Grants-in-Aid from the Ministry of Education, Science, Sports and Culture, Japan (Y.M., Y.K. and T.T.).
These results have been published as follows:
Matsuoka Y., Kitamura Y., Okazaki M., Kakimura J., Tooyama I., Kimura H. and Taniguchi T. (1998) Kainic acid induction of heme oxygenase in vivo and in vitro. Neuroscience, 85: 1223-1233. -> Medline
Matsuoka Y., Kitamura Y., Okazaki M., Sakata M., Tsukahara T. and Taniguchi T. (1998) Induction of heme oxygenase-1 and major histocompatibility complex antigens in transient forebrain ischemia. Journal of Cerebral Blood Flow Metabolism, 18: 824-832. -> Medline
Yasuji
Matsuoka, Ph.D.
Research Associate,
Research Fellow of Japan Society for the Promotion of Science,
Department of Neurobiology,
Kyoto Pharmaceutical University
Yoshihisa Kitamura, Ph.D.
Associate Professor
Department of Neurobiology
Kyoto Pharmaceutical University
Takashi Taniguchi, Ph.D.
Professor and Chairman
Department of Neurobiology
Kyoto Pharmaceutical University
We are welcome for the discussion also for following papers.
**Original Articles:
-Kitamura Y., Kosaka T., Kakimura J., Matsuoka Y., Kohno Y., Nomura Y. and Taniguchi T. (1998) Protective effects of the anti-parkinsonian drugs talipexole and pramipexole against 1-methyl-4-phenylpyridinium-induced apoptotic death in human neuroblastoma SH-SY5Y cells. Molecular Pharmacology, in press. (to be appeared in December issue)
-Matsuoka Y., Kitamura Y., Takahashi H., Tooyama I., Kimura H., Nomura Y. and Taniguchi T. (1998) Interferon-gamma plus lipopolysaccharide induction of delayed neuronal apoptosis in rat hippocampus. Neurochemistry International, in press.
-Matsuoka Y., Kitamura Y., Okazaki M., Terai K. and Taniguchi T. (1998) Kainate-induced activation of nuclear factor-kappaB in rat hippocampus. Experimental Brain Research, in press.
-Kitamura Y., Ota T., Matsuoka Y., Okazaki M., Kakimura J., Tooyama I., Kimura H., Shimohama S., Gebicke-Haerter P.J., Nomura Y. and Taniguchi T. (1998) Kainic acid-induced neuronal loss and glial changes in the hippocampal CA3 of p53-deficient mouse. Neuroscience Letters, in press.
-Kitamura Y., Ota T., Matsuoka Y., Tooyama I., Kimura H., Shimohama S., Nomura Y., Gebicke-Haerter P.J. and Taniguchi T. (1998) Hydrogen peroxide-induced apoptosis mediated by p53 protein in glial cells. Glia, in press.
-Matsuoka
Y., Kitamura Y. and
Taniguchi T. (1998) Induction of plasminogen in rat hippocampal
pyramidal neurons by kainic acid. Neuroscience Letters,
-Matsuoka Y., Kitamura Y., Okazaki M., Sakata M., Tsukahara T. and Taniguchi T. (1998) Induction of heme oxygenase-1 and major histocompatibility complex antigens in transient forebrain ischemia. Journal of Cerebral Blood Flow and Metabolism, 18(8) 824-832. -> Medline
-Matsuoka Y., Kitamura Y., Okazaki M., Kakimura J., Tooyama I., Kimura H. and Taniguchi T. (1998) Kainic acid induction of heme oxygenase in vivo and in vitro. Neuroscience, 85(4) 1223-1233. -> Medline
-Kitamura Y., Shimohama S., Kamoshima W., Matsuoka Y., Nomura Y., Perry G., Whitehouse P.J. and Taniguchi T. (1998) Alternation of proteins regulating apoptosis, Bcl-2, Bcl-x, Bax, Ich-1 and CPP32, in Alzheimer's disease. Brain Research, 780(2) 260-269. -> Medline
-Kitamura Y., Furukawa M., Matsuoka Y., Tooyama I., Kimura H. and Taniguchi T. (1998) In vitro and in vivo induction of heme oxygenase-1 in rat glial cells: possible involvement of nitric oxide production from inducible nitric oxide synthase. Glia, 22(2) 138-148. -> Medline
-Matsuoka Y., Kitamura Y., Fukunaga R., Shimohama S., Nabeshima T., Tooyama I., Kimura H. and Taniguchi T. (1997) In vivo hypoxia-induced neuronal damage in dentate gyrus of rat hippocampus: Changes in NMDA receptors and the effect of MK-801. Neurochemistry International, 30(6) 533-542. -> Medline
-Kitamura Y., Shimohama S., Kamoshima W., Matsuoka Y., Nomura Y. and Taniguchi T. (1997) Changes of p53 in the brains of patients with Alzheimer's disease. Biochemical and Biophysical Research Communications, 232(2) 418-421. -> Medline
-Matsuoka Y., Kitamura Y., Tooyama I., Kimura H. and Taniguchi T. (1997) In vivo hypoxia-induced neuronal damage with an enhancement of neuronal nitric oxide synthase in hippocampus. Experimental Neurology, 146(1) 57-66. -> Medline
-Kitamura Y., Shimohama S., Ota T., Matsuoka Y., Nomura Y. and Taniguchi T. (1997) Alternation of transcription factors, NF-kappaB and STAT1, in the brains of patients with Alzheimer's disease. Neuroscience Letters, 237(1) 17-20. -> Medline
**Review Article & Book:
-Kitamura Y., Matsuoka Y., Nomura Y. and Taniguchi T. (1998) Induction of inducible nitric oxide synthase and heme oxygenase-1 in rat glial cells. Life Sciences, 62(17-18) 1717-1721. -> Medline
-Matsuoka Y., Kitamura Y., Tsukahara T., and Taniguchi T. (1998) Induction of heme oxygenase after transient forebrain ischemia. In: Ischemic Neuronal Cell Death, (eds. Kanazawa I., Yoshioka R. and Yamashita J.), pp. 45-49, SciMed Publications, Tokyo. (in Japanese)