| INABIS '98 Home Page | Your Symposium | Related Symposia & Posters | Scientific Program | Exhibitors' Foyer | Personal Itinerary | New Search |
Introduction
Human essential hypertension is characterised by cardiac hypertrophy and by structural changes in resistance arteries which are often thought to be the result of an adaptive response to the high blood pressure [1]. However, since cardiac hypertrophy is independently associated with sudden death the aim of antihypertensive treatment and also of all drugs that affect the heart and blood vessels should ideally be to reduce cardiac hypertrophy and to reverse the structural changes in resistance arteries so that structure is appropriate to the lower BP. The remodeling of resistance arteries in human essential hypertension and rat genetic hypertension, which results in an increase in the haemodynamically important ratio of the thickness of the tunica media to the lumen diameter (commonly referred to as the media/lumen ratio) can be reversed or ameliorated by antihypertensive treatment, but it appears that some classes of antihypertensive drugs are more effective than others in achieving regression of cardiovascular structure to normal [2-13].
In the New Zealand genetically hypertensive (GH) rat strain the blood pressure (BP) is raised from an early age [14,15] and by the age of 4 weeks, left ventricular (LV) mass is increased and, in mesenteric resistance arteries (MRA), there is structural remodeling leading to an increased media/lumen ratio and a hypertrophy (rather than hyperplasia) of the smooth muscle (SM) cells [15]. The changes in cardiovascular structure have conventionally been thought to be an adaptive response to the raised BP. In this study, our aim was to see if we could prevent the occurence of further structural changes in heart and resistance arteries by treating groups of GH rats for 6 weeks with either an an angiotensin converting enzyme (ACE) inhibitor, enalapril, an angiotensin II (AT1) receptor antagonist, losartan, or a dihydropyridine calcium antagonist, felodipine and to compare their effects on BP, the abnormal cardiovascular structure and on resistance artery remodeling.
Since angiotensin is thought to be involved in the hypertrophy of the smooth muscle (SM) cells of the media, we expected that enalapril and losartan would have a greater effect on cardiovascular structure than would felodipine.
Methods
We used genetically hypertensive rats (GH) rats from the breeding colonies in the Department of Pharmacology, direct descendents of the original GH strain developed by Smirk and colleagues in the 1950s [14,16] and now in their 100th generation. GH (n=10-11 per group) were treated from age 4-10 weeks with enalapril (10mg/kg/d in drinking water) or losartan (15mg/kg/d in drinking water) or felodipine (0.5mg/g chow, to give 30mg/kg/d). The doses of enalapril and losartan were chosen to give equivalent BP lowering and the felodipine dose we have found to have the maximum effect on GH blood pressure. Each treated GH group had untreated GH groups as controls. A single group of normotensive (N) outbred albino control rats, the strain from which the GH rats were originally derived, were common to the losartan and enalapril treated groups, where the drug was given in the drinking water, while the felodipine treated group had its own N control group.
BP (tail-cuff) and body weight were measured weekly and intra-arterial (ia) BP (femoral artery) at the end of the experiment. The heart was removed, the left ventricle weighed and LV mass related to body weight was calculated.
The mesenteric arcade was perfused via the aorta (at a pressure equivalent to the SBP of the animal) first with 75% Tyrode [17] containing heparin (25 000IU; 0.08ml/100ml) per ml and papaverine (2mg/ml) and then with 2% glutaraldehyde in 75% Tyrode. 2nd order branches were dissected out and dehydrated and embedded in Technovit. Sections were cut and stained with a methylene blue- Azur II- basic fuchsin stain for stereological analysis by the method of Cavalieri [18] (for media & lumen volume & percentage of SM within media) and the optical disector [19] (to count SM cell nuclei as a measure of SM cell density). The haemodynamically important ratio of media width/lumen diameter (M/L) and the mean SM cell volume were then calculated.
The INSTAT statistical program (Graphpad software, San Diego, Ca. USA) was used to perform ANOVA, followed by the Bonferroni post-test to test differences between the means. Regression analysis was used where appropriate.
Results
Blood pressure and LV mass
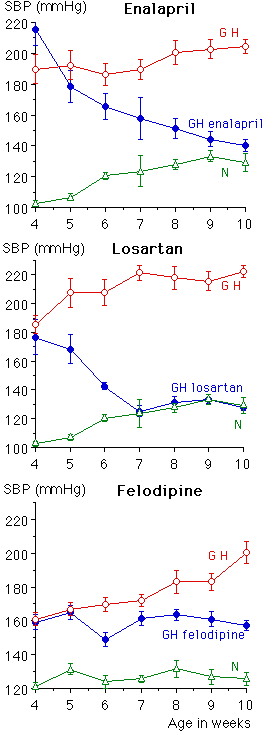
Tail-cuff systolic blood pressure (Figure 1) was reduced significantly to normotensive (N) levels by enalapril and losartan and to a value midway between GH and N control groups by felodipine (Tables 1-3). Intra-arterial mean BP values (not shown) followed a similar pattern . LV mass was significantly reduced by all three drugs. There was a strong relationship between BP and LV mass in the enalapril and losartan groups (r=0.941, p<0.0001 and r=0.78, p< 0.003 respectively) but in the GH felodipine group there was no significant relationship (r=0.329, p=0.16).

Fig. 1: Systolic blood pressure (mmHg, tail-cuff) in all groups from age 4 weeks, when treatment was started, until age 10 weeks.
Table 1. Blood pressure at 10 weeks, left ventricular (LV) mass and structural measurements in mesenteric resistance arteries in the group treated with enalapril
|
|
SBP at 10 weeks, mmHg |
LV mass, mg/100g BW |
Media width, µm |
Lumen diameter, µm |
Ratio, media width/lumen diameter, % |
Media volume µm3/µm |
|
GH control (n=10) |
|
|
|
|
|
|
|
GH enalapril (n=10) |
|
|
|
|
|
|
|
N control (n=10) |
|
|
|
|
|
|
|
GH v N |
|
|
|
|
|
|
|
GH v GH enalapril |
|
|
|
|
|
|
|
N v GH enalapril |
|
|
|
|
|
|
Values in all tables are mean±sem. ANOVA followed by the Bonferroni post-test was used to test the significance of differences between the means.
Table 2. Blood pressure at 10 weeks, left ventricular (LV) mass and structural measurements in mesenteric resistance arteries in the group treated with losartan
|
|
SBP at 10 weeks, mmHg |
LV mass, mg/100g BW |
Media width, µm |
Lumen diameter, µm |
Ratio, media width/lumen diameter, % |
Media volume µm3/µm |
|
GH control (n=10) |
|
|
|
|
|
|
|
GH losartan (n=10) |
|
|
|
|
|
|
|
N control (n=10) |
|
|
|
|
|
|
|
GH v N |
|
|
|
|
|
|
|
GH v GH losartan |
|
|
|
|
|
|
|
N v GH losartan |
|
|
|
|
|
|
Table 3. Blood pressure at 10 weeks, left ventricular (LV) mass and structural measurements in mesenteric resistance arteries in the group treated with felodipine.
|
|
SBP at 10 weeks, mmHg |
LV mass, mg/100g BW |
Media width, µm |
Lumen diameter, µm |
Ratio, media width/lumen diameter, % |
Media volume µm3/µm |
|
GH control (n=11) |
|
|
|
|
|
|
|
GH felodipine (n=9) |
|
|
|
|
|
|
|
N control (n=10) |
|
|
|
|
|
|
|
GH v N |
|
|
|
|
|
|
|
GH v GH felodipine |
|
|
|
|
|
|
|
N v GH felodipine |
|
|
|
|
|
|
Structure of mesenteric resistance arteries.
As we have noted in the past there was hypertrophic remodeling (using the terminology suggested by Mulvany et al [20]) and raised media/lumen ratio in MRA in GH compared with N rats. Enalapril and losartan treatment both significantly reduced the media to lumen ratio by eutrophic outward remodeling ; to N levels in the enalapril group and to below N levels in the losartan-treated group, i.e lumen diameter was increased by 14% in the enalapril group and by 49% in the losartan group, media width decreased by 32% after enalapril and 46% after losartan, but there was no change in unit media volume (per mm3) (Tables 1,2). In the felodipine-treated GH there was a 30% decrease in media width compared with GH control, no change in lumen diameter and significant reduction in media volume by 26%. Thus the ratio in felodipine-treated GH was reduced to N level by hypotrophic remodeling (Table 3).
In all groups regression analysis showed a significant relationship between BP and media/lumen ratio (enalapril r=0.89, p=0.0008; losartan r=0.81, p<0.0001; felodipine r=0.55, p=0.011)
The density of SM cells per unit volume of SM was significantly increased (but not normalised) by all drugs, and the mean volume of the SM cells was significantly reduced. (Table 4). Thus the hypertrophy of the SM cells characteristic of the GH strain was reduced.
Table 4. Smooth muscle cell density and mean smooth
muscle cell volume in media of mesenteric resistance arteries.
|
Enalapril |
|
|
|
GH control (n=10) |
|
|
|
GH enalapril (n=10) |
|
|
|
N control (n=10) |
|
|
|
GH v N |
|
|
|
GH v GH enalapril |
|
|
|
N v GH enalapril |
|
|
|
Losartan |
|
|
|
GH control (n=10) |
|
|
|
GH losartan (n=10) |
|
|
|
N control (n=10) |
|
|
|
GH v N |
|
|
|
GH v GH losartan |
|
|
|
N v GH losartan |
|
|
|
Felodipine |
|
|
|
GH control (n=10) |
|
|
|
GH felodipine (n=10) |
|
|
|
N control (n=10) |
|
|
|
GH v N |
|
|
|
GH v GH felodipine |
|
|
|
N v GH felodipine |
|
|
Discussion
In GH rats treated with enalapril and losartan, BP was lowered significantly to the level of the normotensive N control group, while felodipine treatment lowered BP significantly but not to N levels. LV mass was however significantly reduced to normotensive N values in all groups in spite of the fact that the GH felodipine group did not have their BP reduced to normal. Genetic studies have shown that, in GH, heart mass and BP have separate genetic determinants [21] and it is therefore interesting that in these studies, with the exception of the felodipine treated groups, there is a strong relationship between BP and LV mass. The reduction in LV mass following treatment, was seen even in the felodipine treated groups where the BP was significantly above N level, and suggests that LV mass is to some extent at least independent of BP.
Treatment with enalapril and losartan caused reduction of the media to lumen ratio by remodeling of MRA with the same amount of media being rearranged around an enlarged lumen (eutrophic outward remodeling). The degree of remodeling was greater in the losartan treated GH group. Since both enalapril and losartan treatment resulted in the same kind of structural remodeling it appears that the structural changes are probably due to the blocking of angiotensin rather than any bradykinin effect.
Felodipine treatment led to both a loss of media and enlargement of lumen (hypotrophic outward remodeling) and the reduction in LV mass and structural changes in MRA, giving normalisation of the M/L ratio, occurred even when BP was not fully normalised which suggests that the structural changes may be not wholly dependent on BP.
We have previously reported, using stereological and myograph techniques, the effects on BP and cardiovascular structure of enalapril treatment at twice the dose used here [6,7], and of treatment with the angiotensin II (AT1) receptor antagonist, valsartan (stereological studies only) at three dose levels [8], and it is obvious from reference to those studies together with the results reported here, that in GH the effects of both drug classes are dose dependent; in an earlier study where enalapril was given to GH rats at a dose of 20mg/kg/d there was a greater fall in BP than was found in this study with GH on a dose of 10mg/kg/d, lumen diameter was increased 3-fold on the higher dose compared with the GH control group and there was significant reduction in media volume [6], i.e hypotrophic outward remodeling rather than the eutrophic outward remodeling reported here with the lower dose of enalapril. This dose-dependent effect on structure after ACE treatment has also been shown in myograph studies on resistance artery structure in SHR treated from 4-24 weeks with the ACE inhibitor perindopril [22] but was not seen in another series where SHR were treated with lisinopril (4-20 weeks) [23]. Myograph studies have shown that shown that perindopril, given to SHR from age 6-10 weeks [24] and from 5-10 weeks [25], will lower BP to normal levels, reduce cardiac hypertrophy, and also reduce the media/lumen ratio in mesenteric resistance arteries. The angiotensin II (AT1) receptor antagonists, with their direct action on angiotensin II and therefore growth, should theoretically have more effect on abnormal vascular smooth muscle growth than ACE inhibitors. They have been shown to reduce BP and cardiac hypertrophy effectively in SHR in both young and older rats [11,26,27] to the same extent as we have shown in GH rats given losartan. In losartan treated SHR there was a reduction in media/lumen ratio after 10 weeks treatment (age 3-13 weeks) [26], but there no effect was observed on vessel structure after a shorter period of treatment from age 3-7 weeks [26]. It has recently been reported that SHR given enalapril (25mg/kg/d) and losartan (15mg/kg/d) both outwardly remodeled mesenteric resistance arteries at those doses although BP was lowered less by losartan treatment [28].
These studies concerning the prevention of further cardiovascular structural changes in young GH rats have shown that treatment with enalapril, losartan and felodipine will reduce the raised media/lumen ratio, characteristic of the GH strain from as early as 4 weeks of age [15,29], but, by remodeling (in a different way after felodipine compared with enalapril or losartan), hold it at a level not significantly different from the N control strain. It could be said that the MRA structure in the treated groups had adapted to that required by the new BP level by preventing further abnormal media growth, or in the case of felodipine, reducing media growth. This is not altogether a surprising finding considering the in-vitro findings that calcium antagonists will inhibit angiotensin II-induced cell growth to a greater extent than ACE inhibitors [30] and might be expected to have beneficial effects on development and regression of abnormal cardiovascular growth in hypertension.
Of the three drugs used here, felodipine in fact remodeled the media and lumen structure of GH MRA to a structure closest to their N control group as well as reducing ventricular hypertrophy. This then raises the question of whether it may be a better drug to use than enalapril or losartan. However, losartan and enalapril are both very effective in lowering the media/lumen ratio in GH to normal, as well as reversing ventricular hypertrophy. If, as stated by Folkow [1], the haemodynamically important parameter is the media/lumen ratio since when the media thickens, lumen diameter is reduced and total peripheral resistance increases according to the Poisseuilles equation [1], then the fact that the ratio is lowered by treatment may be the most important factor, irrespective of the exact nature of the remodeling.
In human essential hypertension, resistance arteries show eutrophic inward remodeling [31], therefore antihypertensive treatment should ideally lead to outward remodeling to 'normalise' structure i.e. the effect noted here with enalapril and losartan.
Acknowledgements
This work was supported by the Lottery Health Research Board of New Zealand and the Laurenson Fund of the Otago Medical Research Foundation. The authors wish to thank Mrs Gwen Boullé for technical assistance.
We also wish to thank Merck Sharp and Dohme (NZ Ltd), for arranging supplies of enalapril and losartan and Astra Pharmaceuticals (NZ Ltd), for providing us with felodipine.
References
1. Folkow B. Physiological aspects of primary hypertension. Physiological Reviews 1982, 62: 347-504.
2. Schiffrin EL, Deng LY, Larochelle P. Effects of a ß-blocker or a converting enzyme inhibitor on resistance arteries in essential hypertension Hypertension 1994;23: 83-91.
3. Thybo NK, Stephens N, Cooper A, Aalkjaer C, Heagerty AM, Mulvany MJ. Effect of antihypertensive treatment on small arteries of patients with previously untreated essential hypertension. Hypertension 1995; 25(pt 1): 474-481.
4. Rizzoni D, Muiesan ML, Porteri E, Castellano M, Zulli G, Salvetti M, Minteduro C, Agabiti Rosei E. Effect of long-term antihypertensive treatment with lisinopril on resistance arteries in hypertensive patients with left ventricular hypertrophy. Journal of Hypertension 1997; 15: 197-204.
5. Korsgaard N, Christensen KL, Mulvany MJ. Cellular morphology in mesenteric resistance vessels from antihypertensive treated spontaneously hypertensive rats. Basic Research in Cardiology 1991;86 (suppl 1):33-41.
6. Ledingham JM, Phelan EL, Cross M, Laverty R, Millar JA. Remodelling of resistance arteries by treatment with enalapril in the New Zealand genetically hypertensive GH) rat. Clinical and Experimental Pharmacology and Physiology 1994; 21:235-237
7. Ledingham JM, Laverty R. The long-term effects of treatment with enalapril on structure of mesenteric resistance arteries in New Zealand genetically hypertensive rats. Clinical and Experimental Pharmacology and Physiology 1995; 22 (Suppl 1): S350-S352.
8. Ledingham JM, Laverty R. Remodelling of resistance arteries in genetically hypertensive rats by treatment with valsartan, an Angiotensin II receptor antagonist. Clinical and Experimental Pharmacology and Physiology 1996, 23: 576-578.
9. Lee RMKW, Berecek KH, Tsoporis J, McKenzie R, Triggle CR. Prevention of hypertension and vascular changes by captopril treatment. Hypertension 1991; 17: 141-50.
10. Lundin SA, Hallbäck-Nordlander MIL. Regression of structural cardiovascular changes by antihypertensive therapy in spontaneously hypertensive rats Journal of Hypertension 1984; 2:11-18.
11. Oddie CJ, Dilley, Kanellakis P, Bobik A. Chronic angiotensin II type receptor antagonism in genetic hypertension: effects on vascular structure and reactivity. Journal of Hypertension 1993; 11: 717-724.
12. Porteri E, Rizzoni D, Castellano M, Bettoni G, Muiesan ML Salvetti M, Quartaroli M, Gaviraghi G, Agabiti Rosei E. Structural changes of small resistance arteries in spontaneously hypertensive rats after treatemnt with various doses of lacidipine. Journal of Hypertension 1997, 15: 619-625.
13. Rizzoni D, Castellano M, Porteri E, Bettoni G, Muiesan ML, Cinelli A, Agabiti Rosei E. Effects of low and high doses of fosinopril on the structure and function of resistance arteries. Hypertension 1995; 26: 118-123.
14. Simpson FO, Phelan EL, Ledingham JM, Millar JA. Hypertension in the genetically hypertensive (GH) strain. In: Ganten D, de Jong W, eds. Handbook of Hypertension, Vol 16: Experimental and genetic models of hypertension. Amsterdam: Elsevier Science BV, 1994; 228-271.
15. Ledingham JM, Millar JA. Stereological studies on mesenteric resistance artery structure in NZ genetically hypertensive rat (GH) and their control (N) rats. Clinical and Experimental Pharmacology and Physiology 1993; 20: 359-361.
16. Smirk FH and Hall WH: Inherited hypertension in rats. Nature (London) 1958, 182:727-728.
17. Maunsbach AB. The influence of different fixatives and fixation methods on the ultrastructure of rat kidney tubules. Journal of Ultrastructure Research 1966;15: 283-309.
18. Gundersen HJG, Bendtsen TF, Korbo L, Marcussen N, Moller A, Nielsen K, Nyengaard JR, Pakkenberg B, Sorensen FB, Vesterby A, West MJ. Some new, simple and efficient stereological methods and their use In: Pathological Research and Diagnosis. Acta Pathologica Microbiologica et Immunologica Scandinavica 1988; 96: 379-394.
19. Gundersen HJG, Bagger P, Bendtsen TF, Evans SM, Korbo L, Marcussen N, Moller A, Nielsen K, Nyengaard JR, Pakkenberg B, Sorensen FB, Vesterby A, West MJ. The new stereological tools: disector, fractionator, nucleator and point sampled intercepts and their use in pathological research and diagnosis. Acta Pathologica Microbiologica et Immunologica Scandinavica 1988, 96: 857-881.
20. Mulvany MJ, Baumbach GL, Alkjaer C, Heagherty AM, Korsgaard N, Schiffrin EL, Heistad DD. Vascular remodeling. Hypertension 1996; 28: 505-506.
21. Harris EL, Phelan EL, Thompson CM, Millar JA, Grigor MR. Heart mass and blood pressure have separate genetic determinants in the New Zealand genetically hypertensive (GH) rat. Journal of Hypertension 1995; 13: 397-404.
22. Thybo NK, Korsgaard N, Eriksen S, Christensen KL, Mulvany MJ.Dose-dependent effeects of perindopril on blood pressure and small-artery structure. Hypertension 1994; 23: 659-666.
23. Shaw LM, George PR, Oldham AA, Heagherty AM. A comparison of the effect of angiotensin converting enzyme inhibition and angiotensin II receptor antagonism on the structural changes associated with hypertension in rat small arteries. Journal of Hypertension 1995; 13: 1135-1143.
24. Harrap SB, Van der Merwe WM, Griffin SA, Macpherson F, Lever AF. Brief angiotensin converting enzyme inhibitor treatment in young spontaneously hypertensive rats reduces blood pressure long-term. Hypertension 1990; 16: 603-614.
25. Harrap SB, Mitchell GA, Casley DJ, Mirakian S, Doyle AE. Angiotensin II, sodium, and cardiovascular hypertrophy in spontaneously hypertensive rats. Hypertension 1993; 21: 50-55.
26. Morton JJ, Beattie EC, MacPherson F. Angiotensin II receptor antagonist losartan has persistent effects on blood pressure in the young spontaneously hypertensive rat: lack of relation to vascular structure. Journal of Vascular Research 1992; 29: 264-269.
27. Soltis EE. Alterations in vascular structure and function after short-term losartan treatment in spontaneously hypertensive rats. J Pharmacol exp Ther 1993: 266: 642-646.
29. Phelan EL, Cross M, Millar JA. Early structural changes in mesenteric resistance arteries of (MRA) of New Zealand genetically hypertensive (GH) rats. J Hypertension 1994; 12 (suppl 3): S214.
29. Rizzoni D, Porteri E, Piccoli A, Castellano M, Bettoni G, Muiesan ML, Pasini G, Guelfi D, Mulvany MJ, Agabiti Rosei E. Effects of losartan and enalapril on small artery structure in hypertensive rats. Hypertension 1998; 32: 305-310.
30. Sachinidis A, Ko Y, Graack GKH, Wieczorek AJ, Vetter H. Action of metoprolol, enalapril, diltiazem, verapamil and nifedipine on cell growth of vascular smooth muscle cells. Journal of Cardiovascular Pharmacology 1992; 19 (suppl 1): S60-S62.
31. Heagherty AM, Aalkjaer C, Bund SJ,Korsgaard N, Mulvany MJ. Small artery structure inhypertension: dual process of remodeling and growth. Hypertension 1993; 21: 391-397.