Full Text Page
ROLE OF THE MESOLIMBIC CHOLINERGIC PATHWAYS IN THE
INITIATION OF VOCALIZATION IN CATS AND RATS
Stefan M. Brudzynski
Department of Psychology, Brock University
St. Catharines, Ontario, L2S 3A1 Canada
E-mail: sbrudzyn@spartan.ac.brocku.ca
ABSTRACT
The vocal component of defensive behaviour, with other accompanying
manifestations, may be reproduced by an electrical or chemical stimulation of the brain.
Results of studies during the last 10 years have demonstrated that the defensive or alarm
vocalizations may be induced by cholinergic, muscarinic stimulation of the homolog areas
of cat and rat brains. These cholinoceptive muscarinic regions occupy in both these
species an elongated medial strip of tissue from the brainstem periaqueductal grey, medial
tegmental regions, medial hypothalamic-preoptic and periventricular regions, up to the
mediobasal forebrain and septal structures. The following presentation summarizes results
of several recent studies which demonstrate that the ascending mesolimbic cholinergic
projection from the laterodorsal tegmental nucleus is responsible for triggering the
ultrasonic alarm calls (22 kHz calls) in adult rats. It is suggested that this mesolimbic
cholinergic projection plays a similar role in the cat's brain. Release of acetylcholine
from the mesolimbic cholinergic terminals distributed predominantly along the medial
limbic structures, causes a dose dependent postsynaptic inhibition of neuronal firing. It
is postulated that this vast inhibitory response represents a trigger for the behavioural
response and alarm or threatening vocalization.
INTRODUCTION
Vocalization accompanying defensive behaviour is produced in a
species-specific way, depending on the biology of the species, its social structure and
behavioural situation. For instance, vocalization may be emitted as a threatening call,
like growling vocalization in the cat, which is usually addressed to a single opponent, or
as a ultrasonic alarm call, like 22 kHz calls emitted by rats and usually addressed to
many individuals in the colony. Despite of these differences, however, it seems that the
defensive calls are controlled by a common neural and neurochemical substrate and may be
reproduced by electrical or chemical stimulation of the brain. Defensive or alarm
vocalizations can be induced by muscarinic cholinergic stimulation of the homolog areas of
the cat and rat brains.
RESULTS AND DISCUSSION
Species-specific growling type of vocalization have been induced in
cats by intracerebral carbachol from an elongated, but limited strip of medial structures,
from the periaqueductal gray, through the medial hypothalamic and preoptic regions, to the
septal nuclei, and intralaminar thalamic nuclei (Baxter 1967, 1968; Brudzynski &
Eckersdorf 1988; Brudzynski et al. 1995; Decsi 1974; Decsi & Nagy 1977; Myers 1964;
Varszegi & Decsi 1967). A diagram of this system compiled from several studies is
illustrated on a midsagittal section of the cat brain in Fig.1.
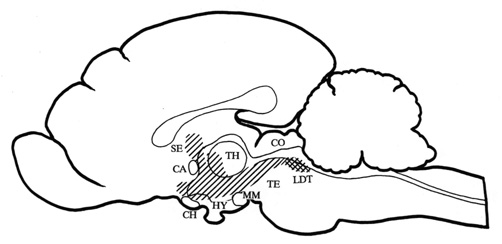
Fig. 1. Midsagittal section through the cat brain with the
cholinoceptive strip of medial structures (hatched area) from which the local application
of carbachol induced behavioural response with the growling type vocalization as its main
manifestation. The strip includes periaqueductal gray, medial tegmentum, medial midbrain
reticular formation, zona incerta, posterior and doral hypothalamic regions, perifornical
hypothalamic region, para- and pariventricular hypothalamic nuclei, anterior
hypothalamic-preoptic area, nucleus of the diagonal band, nucleus of commissure anterior,
septal nuclei, and intralaminar thalamic nuclei. The diagram has been compiled from data
obtained from Baxter 1967, Brudzynski et al. 1995, Decsi 1974, and Decsi & Nagy 1977.
The cholinergic innervation originates from the LDT nucleus (cross hatched area).
Abbreviations: CA - commissura anterior, CH - optic chiasm, CO - colliculi, HY -
hypothalamus, LDT - laterodorsal tegmental nucleus, MM - mammillary bodies, SE - septum,
TH - thalamus, TE - tegmentum.
Intracerebral application of carbachol into the rat brain was also
reported to induce vocalization, which was indistinguishable from the naturally occurring
22 kHz ultrasonic alarm calls (Brudzynski & Bihari 1990). Functional mapping of the
response induced by carbachol from the rat brain delineated a similar brain system to that
one in the cat brain (Brudzynski 1994; Dencev et al. 1996). A similar strip of medially
located regions from the tegmentum to the preoptic area and septum has been revealed. A
diagram of this cholinoceptive strip of structures is shown on a midsagittal section of
the rat brain in Fig. 2.
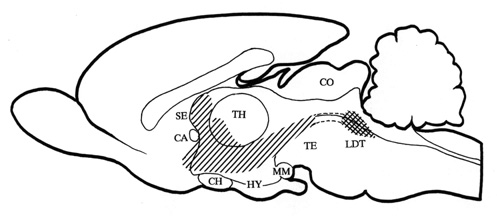
Fig. 2. Midsagittal section through the rat brain
with the cholinoceptice strip of medial structures (hatched area) from which the local
application of crabachol induced behavioural response with the 22 kHz type of alarm calls
as its main manifestation. The strip includes rostral reticular formation, prerubral
field, zona incerta, dorsal hypothalamus, para- and pariventricular hypothalamic nuclei,
medial hypothalamic area, anterior hypothalamic-preoptic area, diagonal band of Broca,
medial-ventral pallidum, anteromedial nucleus accumbens, and septum. The diagram has been
compiled from data obtained from Brudzynski 1994, Dencev et al. 1996, and Dencev &
Brudzynski - unpublished observations). The cholinergic innervation originates from the
laterodorsal tegmental nucleus (LDT) (cross hatched area). Abbreviations: see legend to
Fig. 1.
The patterns of cholinoceptice structues shown in Fig. 1 and 2 are
strikingly similar to the pattern of the ascending projections from the pontomesencephalic
cholinergic neurons (Satoh & Fibiger 1986; Woolf et al. 1990). This group of
cholinergic neurons is localized within the pedunculopontine, parabrachial, and
latrodorsal tegmental nuclei (LDT) (Armstrong et al. 1983; Kimura et al. 1981; Lauterborn
et al. 1993; Mesulam et al. 1989). The ascending component of the cholinergic innervation,
however, originates predominantly from the LDT nucleus.
It has been shown in a behavioural-pharmacological study in rats that
chemical stimulation of the LDT nucleus with glutamate induced 22 kHz alarm calls which
were similar to those obtained by carbachol from the basal forebrain regions (Brudzynski
& Barnabi 1996). Glutamate has a strong, non-specific excitatory effect on neuronal
cell bodies and its application into the LDT activated cholinergic neurons within this
nucleus. The LDT neurons have extensive ascending projections and innervate numerous
nuclei in the thalamus, hypothalamus, basal forebrain, septum, and basal ganglia (Cornwall
et al. 1990; Satoh & Figiber 1986). Activiation of these cholinergic cells was
reported to release acetylcholine in the basal forebrain (Consolo et al. 1990). Thus,
stimulation of these cholinergic cells with glutamate caused release of acetylcholine in
most of the nuclei of the cholinoceptive vocalization system and triggered ultrasonic
alarm vocalization (Brudzynski & Barnabi 1996).
In order to demonstrate that this mechanism is responsible for the
initiation of vocalization, the 22 kHz calls have been induced by an intra-LDT injection
of glutamate and this response was antagonized by the local application of scopolamine, a
muscarinic antaginist, into the hypothalamic-preoptic area. The medial
hypothalamic-preoptic area is a significant portion of the terminal field of the
cholinoceptive vocalization strip and receives cholinergic innervation from the LDT
nucleus (Satoh & Fibiger 1986). The pretreatment of the anterior hypothalamic-preoptic
area with scopolamine significantly decreased the number of ultrasonic calls and the total
duration of the response induced by glutamate from the LDT (Fig. 3).
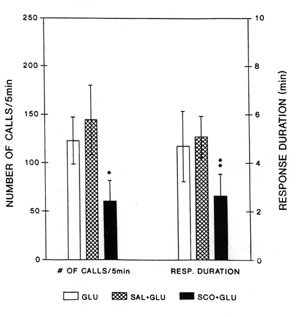
Fig. 3. Average number of 22 kHz alarm calls
(left three bars) and average duration of the vocal response (right three bars) induced by
injection of glutamate into the laterodorsal tegmental nucleus (LDT) with different
pretreatments. Blank bars: response after injection of L-glutamate (GLU) without
preteratment; Hatched bars: L-glutamate-induced response after bilateral preteratment of
the anterior hypothalamic-preoptic area with saline (2 x 0.2 m
l, SAL + GLU); Black bars: L-glutamate-induced response after bilateral pretreatment of
the anterior hypothalamic-preoptic area with (-)-scopolamine (2 x 2 m
g in 2 x 0.2 m l, SCO + GLU). Vertical lines represent SEMs.
Number of calls and response duration after pretreatment with scopolamine were
significantly attenuated as compared with those after saline pretreatment (Wilcoxon signed
rank test: * - P < 0.02, and ** - P < 0.006). From Brudzynski & Barnabi 1996).
A similar result has been recently replicated with the scopolamine
pretreatment of the septum (Dencev & Brudzynski, unpublished data).
The cellular mechanism by which the ascending cholinergic inputs
initiate vocalizational responses is not clear. However, it has been found in acute rat
preparation that carbachol caused a decrease in the firing rate of spontaneously active
neurons in the anterior hypothalamic-preoptic area (Brudzynski et al. 1991; 1998). The
deacrease in the firing rate was obtained from a comparable regions to those from which
vocalizational responses had been induced. In a recent study, the decrease in the mean
firing rate of neurons in the anteromedial hypothalamic-preoptic area was replicated by an
electrical stimulation of LDT - the source of the ascending cholinergic projection
(Brudzynski et al. 1998). Single pulse electrical stimulation of the LDT caused
current-dependent inhibitition of firing, which could stop generation of action potentials
in hypothalamic-preoptic neurons for as long as 50 ms. It was also possbile to demonstrate
that tha same neurons which inhibited their firing rate to eletrical stimulation of the
LDT showed also a dose-dependednt inhibition of firing caused by the local extracellular
inotophoresis of carbachol (Fig. 4).
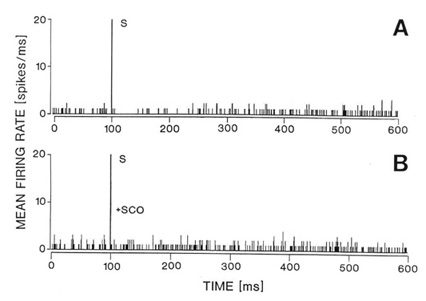
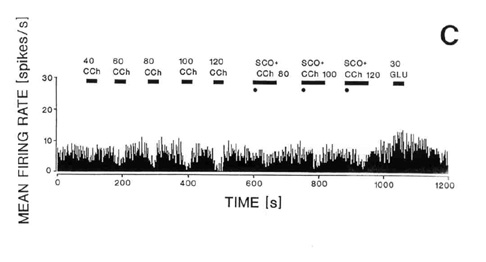
Fig. 4. Responses of the same single neuron in the
naterior hypothalamic-preoptic area to electrical stimulation of the laterodorsal
tegmental nucleus (LDT) (A-B) or to local iontophoresis of carbachol (C). A: Peristimulus
histogram showing that electrical stimulation (S) of the LDT caused inhibition of the
firing rate. B: The inhibition was reversed by local iontophoretic preteratment of the
neuron with scopolamine (+SCOP). C: Iontophoretic application of carbachol (CCh) into the
vicinity of the same neuron caused a dose-dependent (40-120 nA) decrease in the neuron
firing rate as shown on the running time histogram (left side of the histogram). Responses
to iontophoretic carbachol (80-120 nA) were reversed or attenuated by a concurrent local
application of scopolmine (SCO + CCh, right side of the histogram). The same unit
responded with an increase in firing rate to iontophoretic application of glutamate (GLU,
30 nA, far right). For further details of the experiment see the sourse paper, Brudzynski
et al. 1998.
It seems, therefore that a widespread neuronal inhibition caused by
release of acetylcholine from the the mesolimbic cholinergic projection is associated with
the initiation of defensive behaviour with threatening or alarming vocalizations. A number
of studies provided evidence for a behaviour-dependent decrease in the firing rate at
least within the hypothalamic-preoptic area (Adams 1968; Mink et al. 1983; Naka & Kido
1967). On the basis of our results and previous behavioural studies, it is postulated that
the vast inhibitory influence of the ascending choliergic fibres in the mediobasal
forebrain and diencephalon represents a trigger for the behavioural response and alarm or
threatening vocalization.
ACKNOWLEDGEMENTS
The studies have been supported by grants from the Natural Sciences and
Engineering Research Council of Canada.
REFERENCES
Adams, D.B. (1968) The activity of single cells in the midbrain and
hypothalamus of the cat during affective defense behavior. Arch. Ital. Biol. 106:
243-269.
Armstrong, D.M., Saper, C.B., Levey, A.I., Wainer, B.H. and Terry, R.D.
(1983) Distribution of cholinergic neurons in rat brain: demonstrated by the
immunocytochemical localization of choline acetyltransferase. J. Comp. Neurol. 216:
53-68.
Baxter, B.L. (1967) Comparison of the same behavioral effects of
electrical or chemical stimulation applied at the same brain loci. Expt. Neurol.
19: 412-432.
Baxter, B.L. (1968) Elicitation of emotional behavior by electrical or
chemical stimulation applied at the same loci in the cat mesencephalon. Expt. Neurol.
21: 1-10.
Brudzynski, S.M. (1994) Ultrasonic vocalization induced by
intracerebral carbachol in rats: Localization and dose-response study. Behav. Brain
Res. 63: 133-143.
Brudzynski, S.M. and Barnabi, F. (1996) Contribution of the ascending
cholinergic pathways in the production of ultrasonic vocalization in the rat. Behav.
Brain Res. 80: 145-152.
Brudzynski, S.M. and Bihari, F. (1990) Ultrasonic vocalization in rats
produced by cholinergic stimulation of the brain. Neurosci. Lett. 109: 222-226.
Brudzynski, S.M. and Eckersdorf, B. (1988) Vocalization accompanying
emotional-aversive response induced by carbachol in the cat: Reproducibility and a
dose-response study. Neuropsychopharmacology 1: 311-320.
Brudzynski, S.M., Eckersdorf, B. and Golebiewski, H. (1995) Regional
specificity of the emotional-aversive response induced by carbachol in the cat brain. A
quantitative mapping study. J. Psychiatr. Neurosci. 20: 119-132.
Brudzynski, S.M., McLachlan, R.S., Bihari, F. and Girvin, J.P. (1991)
Response of neurons of the rat anterior hypothalamic-preoptic area to intracerebrally
applied carbachol. Brain Res. Bull. 26: 929-934.
Brudzynski, S.M., Kadishevitz, L. and Fu, X-W. (1998) Mesolimbic
component of the ascending cholinergic pathways: Electrophysiological-pharmacological
study. J. Neurophysiol. 79: 1675-1686.
Consolo, S., Bertorelli, R., Forloni, G.L. and Butcher, L.L. (1990)
Cholinergic neurons of the pontomesencephalic tegmentum release acetylcholine in the basal
nuclear complex of freely moving rats. Neuroscience 37: 717-723.
Cornwall, J., Cooper, J.D. and Phillipson, O.T. (1990) Afferent and
efferent connections of the laterodorsal tegmental nucleus in the rat. Brain Res. Bull.
25: 271-284.
Decsi, L. (1974) Behavioral effects of intracerebrally injected
carbachol on unrestrained cats. Pharm. Biochem. Behav. 2: 141-143.
Decsi, L. and Nagy, J. (1977) Adrenergic modulation of a cholinergic
emotional reaction in the cat's thalamus. Psychopharmacology 54: 303-305.
Dencev, A., Hrycyshyn, A.W. and Brudzynski, S.M. (1996) Cholinergic
projection to the basal forebrain involved in the initiation of ultrasonic vocalization in
the rat. Abstr. Int. Behav. Neurosci. Soc. 5: 60.
Kimura, H., McGeer, P.L., Peng, J.H. and McGeer, E.G. (1981) The
central cholinergic system studies by choline acetyltransferase immunohistochemistry in
the cat. J. Comp. Neurol. 200: 151-201.
Lauterborn, J.C., Isackson, P.J., Montalvo, R. and Gall, C.M. (1993) In
situ hybridization localization of choline acetyltransferase mRNA in adult rat brain and
spinal cord. Mol. Brain Res. 17: 59-69.
Mesulam, M.-M., Geula, C., Bothwell, M.A. and Hersh, L.B. (1989) Human
reticular formation: Cholinergic neurons of the pedunculopontine and laterodorsal
tegmental nuclei and some cytochemical comparisons to forebrain cholinergic neurons. J.
Comp. Neurol. 281: 611-633.
Mink, J.W., Sinnamon, H.M. and Adams, D.B. (1983) Activity of basal
forebrain neurons in the rat during motivated behaviors. Behav. Brain Res. 8:
85-108.
Myers, R.D. (1964) Emotional and autonomic responses following
hypothalamic chemical stimulation. Canad. J. Psychol. 18: 6-14.
Naka, K.-I. and Kido, R. (1967) Hypothalamic spike potentials recorded
by chronically implanted tungsten microelectrodes. Brain Res. 5: 422-424.
Satoh, K. and Fibiger, H.C. (1986) Cholinergic neurons of the
laterodorsal tegmental nucleus: Efferent and afferent connections. J. Comp. Neurol.
253: 277-302.
Varszegi, M.K. and Decsi, L. (1967) Some characteristics of the rage
reaction evoked by chemical stimulation of the hypothalamus. Acta Physiol. Acad. Sci.
Hung. 32: 61-68.
Woolf, N.J., Harrison, J.B. and Buchwald, J.S. (1990) Cholinergic
neurons of the feline pontomesencephalon. II. Ascending anatomical projections. Brain
Res. 520: 55-72.
| Discussion Board | Previous Page | Your Symposium
|
Brudzynski, SM;
(1998). Role of the Mesolimbic Cholinergic Pathways in the Initiation of Vocalization in Cats and Rats. Presented at INABIS '98 - 5th Internet World Congress on Biomedical Sciences at McMaster University, Canada, Dec 7-16th. Invited Symposium. Available at URL http://www.mcmaster.ca/inabis98/brudzynski/brudzynski0219/index.html
© 1998 Author(s) Hold Copyright




