David J. Kennaway, Robert W. Moyer and Sally A. Ferguson
It is well established that circadian rhythms of activity, temperature and hormone secretion are generated by the suprachiasmatic nuclei (SCN). When animals are maintained in continuous light or darkness, the periods of the rhythms are determined by the endogenous period of the SCN. In humans and laboratory rats this period is slightly greater than 24 hours. In some other animals, for example mice and some Syrian hamsters, the period may be less than 24 hours. To prevent all of the rhythms free-running an entrainment system exists in vertebrates that utilises the retinal perception of environmental light. Through processes not yet fully understood, but probably involving alterations in gene expression of genes like per, bmal1 and clock, light can change the timing of the output signals generated by the SCN to lock them into the solar day/night cycle.
The entrainment of rhythms is achieved via neural connections from the retina to the SCN. There are a number of pathways through which light can alter SCN function. The predominant pathway is the direct retino-hypothalamic tract (RHT) wherein neurons originating in the retinal ganglion cells project as part of the optic nerve bundle to synapse on cells in the ventro-lateral region of the SCN. The transmitter involved in this pathway is generally believed to be an excitatory amino acid, either aspartate, glutamate or N-acetylaspartylglutamate (NAAG) as well as pituitary adenylate cyclase activating peptide (PACAP). The rat SCN cells contain the mRNA and protein for a large range of excitatory amino acid receptors of both the metabotrophic and ionotrophic type. Another tract which probably involves branching of neurons from the retino-hypothalamic tract projects to the intergeniculate leaflet where they synapse on neurons projecting back to the SCN. The transmitters involved in this pathway, at least in the final projection, are GABA and Neuropeptide Y. Again, GABAA and GABAB as well as Y1 and Y5 receptors for the transmitters are present in SCN cells. A third, putative tract involves a projection of neurones to the dorsal raphe nucleus, which then project to the SCN [1]. The neurons from the raphe use serotonin as the transmitter, but what serotonin receptor sub-type(s) are present and/or functional in the SCN is controversial. Finally, there are cholinergic projections from the brain stem and basal forebrain that terminate in the SCN. It is known that there are retinal projections to these areas, but it is not known whether there is a functional link between the retinal efferents and the SCN afferents.
Our group has been evaluating the effects of light on melatonin rhythms, activity and temperature rhythms and the induction of the immediate early gene c-fos for several years. A particular focus of our studies has involved the neurotransmitters involved in the mediation of the light effects on rhythmicity. This paper reviews our studies on melatonin and other rhythms in the rat over the last few years and presents our views on the impact of light and the role of transmitters on them.
Background
Melatonin rhythms in the rat and unexpected light presentation at night
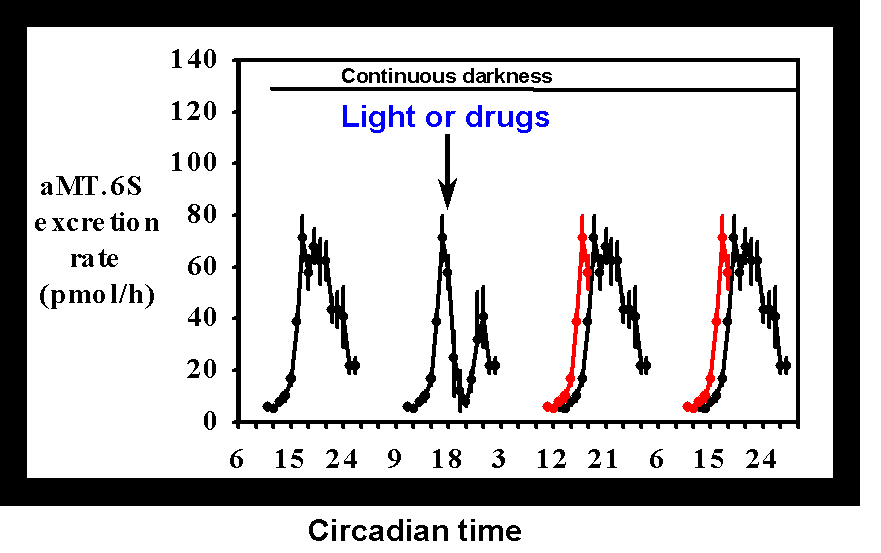
Kennaway (1993) [2] reported a non-invasive procedure for investigating melatonin rhythmicity in individual rats based upon the measurement of the urinary excretion rate of the melatonin metabolite, 6-sulphatoxymelatonin (aMT.6S). The procedure allows melatonin production to be monitored for prolonged periods of time, uses small numbers of animals, allows repeated measures statistical tests to be used, is simple and inexpensive and provides information similar to more invasive and terminal approaches. Figure 1 shows the type of information that can be obtained from the experiments.

Figure 1 The mean
± SEM of the urinary aMT.6S excretion rate for 5 animals maintained in a 12L:12D photoperiod. The excretion rate across 4 nights following release into continuous darkness is shown in the black lines. Gaps are due to failure to urinate during sleep. The red lines plotted on the third and fourth subjective nights are a re-plot of the rising phase of aMT.6S excretion on the second night to emphasise any phase shifts. The arrow indicates the time of a stimulus, in this case a 2 lux/15 minute light pulse.The usual approach in experiments utilising this design is to monitor melatonin rhythmicity on a control night when no intervention is made, followed by a treatment night wherein a light pulse or drug treatment or a drug treatment before a light pulse is administered. In early experiments we used a design whereby the animals were maintained in a LD cycle through out the experiment (Aschoff's Type IV method [3]). Subsequently we have used the Aschoff Type II model whereby the animals are maintained in LD and then released into continuous darkness (DD) and treatments administered on the first subjective night after the imposition of DD. This latter procedure allows us to explore treatments that might be expected to induce phase advances, something that could not be expected to work using the former method due to masking by the LD cycle. While we have not formally compared the two methods, the phase shifts we have observed in response to light have been very similar.
When light was presented at different times of the night to rats (200 lux/ 15 minutes) the onset of melatonin production was acutely inhibited for a few hours and if the light exposure was early in the night, then the melatonin production resumed until dawn; otherwise it remained suppressed. On the next night(s) the onset of melatonin production was differentially delayed depending upon the time of the light presentation [4]. These results were essentially similar to those found by Illnerova [5] using pineal NAT activity measurements. We were interested to find that even when light was presented at ZT 22, ie 2 hours before lights on in the morning, rather than see a phase advance as might be expected from studies on activity rhythms in rats kept for prolonged periods in DD, we found a small phase delay. This type of experiment has provided the cornerstone of our studies on the neurotransmitters involved in mediating the effects of light on melatonin rhythms.
Pharmacological manipulation of melatonin rhythms in the rat
Excitatory amino acids
The first experiments looking at the effects of interactions between light and drugs focussed upon the role of excitatory amino acids, which are putative transmitters of the RHT. Previous research in hamsters had indicated that an antagonist of the N-methyl-D-aspartate receptor, MK-801, could block the effects of a light pulse on activity rhythms and the acute effects of light on pineal melatonin content. In a series of experiments detailed in Rowe and Kennaway (1996) [6] we found that administration of MK-801 (3 mg/kg) prior to a 2lux/1 minute light pulse at ZT16 failed to alter either the acute suppression of melatonin by light or the subsequent phase shift in the onset the next night. We also failed to block the phase shift in running activity induced with a light pulse at the same dose of MK-801. Subsequently we investigated another EAA antagonist which is specific for the kainate type of receptor, DNQX (3 mg/kg) and also failed to block the acute or phase shifting effects of light (Kennaway 1997) [7]. We concluded "our results do not support the hypothesis that EAA in the retino-hypothalamic tract terminating in the SCN --- are the major transmitters mediating the photic influences upon rat pineal and activity rhythms."
Serotonin
When we investigated the effects of serotonergic drugs on pineal melatonin rhythmicity the expectation from in vitro studies of SCN neuronal firing rate rhythms was that serotonin agonists (quipazine, 8-OHDPAT and serotonin) would cause phase advances when administered at ZT6 and either no effect (serotonin and 8-OHDPAT) or a phase delay (quipazine) at ZT13, ZT15 or ZT19. In Kennaway et al (1996) [8] we showed that quipazine (3 mg/kg) administered to rats at CT18 resulted in an acute inhibition of melatonin production for several hours followed by recovery of production until subjective dawn. On the following nights the onset of melatonin production was significantly delayed by an average of 1.5 hours. Treatment with quipazine at either CT6 or CT12 failed to provoke an advance in the onset of melatonin production. Furthermore quipazine administration was found to result in the induction of the immediate early gene c-fos in the ventrolateral region of the SCN in an identical manner to that found with brief light exposure [9]. Administration at ZT6 failed to increase the number of SCN cells immunopositive for c-FOS protein.
What serotonin receptors are involved?
We have subsequently addressed the question of what serotonin receptor sub-types are involved in the mediating the effects of light on rhythms in the rat. To this end we have studied a wide range of serotonergic agonist drugs administered systemically.
Table 1 Effects of various serotonergic drugs, administered at CT18 on the onset of melatonin production.
|
Drug |
Dose |
Receptor Type |
Delay on Night 3 |
Delay on Night 4 |
|
|
|
|
|
|
|
DOI |
0.1 mg/kg |
5-HT2a/2c |
1.2 ± 0.2* |
2.3 ± 0.2* |
|
DOI |
0.5 mg/kg |
5-HT2a/2c |
1.7 ± 0.2* |
2.1 ± 0.2* |
|
MCPP |
2 mg/kg |
5-HT2a/2c |
1.7 ± 0.3* |
1.7 ± 0.4* |
|
TFMPP |
2 mg/kg |
5-HT2a/2c |
1.4 ± 0.8* |
1.5 ± 0.4* |
|
MK-212 |
2 mg/kg |
5-HT2c |
0.7 ± 0.3 |
0.3 ± 0.7 |
|
MK-212 |
9 mg/kg |
5-HT2c |
1.2 ± 0.1* |
1.2 ± 0.3* |
|
Buspirone |
2 mg/kg |
5-HT1a |
0.2 ± 0.2 |
0.5 ± 0.5 |
|
Phenylbiguanide |
10 mg/kg |
5-HT3 |
0.0 ± 0.2 |
0.3 ± 0.4 |
|
R-(+)-8-OH-DPAT (a) |
2 mg/kg |
5-HT7 |
-0.1 ± 0.1 |
0.5 ± 0.1 |
|
Quipazine (b) |
1 mg/kg |
5-HT2c (?) |
0.8 ± 0.1* |
1.5 ± 0.5* |
|
( ± )-8-OH-DPAT (b) |
5 mg/kg |
5-HT1a |
0.5 ± 0.2 |
0.9 ± 0.3 |
|
Saline |
- |
- |
0.3 ± 0.2 |
0.4 ± 0.2 |
The data show the mean
± SEM (hours, n = 5) delay in the onset of aMT.6S excretion on the two nights after administration of the various serotonin agonists sub cutaneously at CT18. * Indicates a significant delay in the onset compared to night 2. (a) This enantiomer has a 20 fold higher affinity for the 5-HT7 receptor than the racemic mixture. (b) The data for these drugs has been recalculated from data in a previous paper (Kennaway et al. (1996) [8]. All other data is from Kennaway and Moyer (1998) [10] or unpublished results.It is clear from the results in Table 1 that the drugs that bring about the phase shifting of the melatonin rhythm are those that are agonists for the 5-HT2a/2c receptor; DOI ((
± )-1-(4-Iodo-2,5-dimethoxyphenyl)-2-aminopropane hydrochloride), mCPP (1-(3-Chlorophenyl)-piperazine), TFMPP (N-(3-Trifluoromethylphenyl)-piperazine HCl). These agonists have very high affinity and specificity for both receptor subtypes. Quipazine is a non specific serotonin agonist that has quite low affinity for all serotonin receptors but interestingly has highest affinity for the 5-HT2c receptor. MK-212 (6-Chloro-2-(1-piperazinyl) pyrazine) is a serotonin agonist with high selectivity for the 5-HT2c receptor but relatively low affinity. This is a likely reason for the higher dose of MK-212 that was required to induce the phase shift.Figure 2 shows the effect of MK-212 (9 mg/kg) on aMT.6S excretion when administered at CT18. After a slight delay, the production of melatonin decreased and then resumed during the late subjective night. On the next two nights the shift in the onset of the melatonin production can be clearly seen.
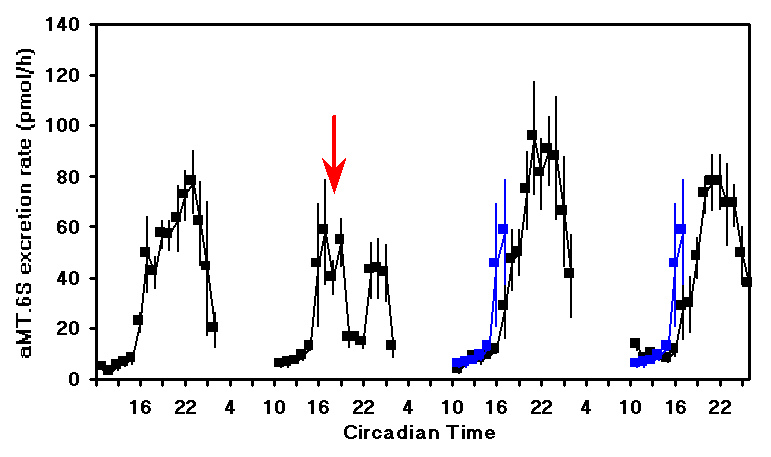
Figure 2. The mean
± SEM of the urinary aMT.6S excretion rate for 5 animals treated on the second subjective night at CT18 (arrow) with MK-212 (9 mg/kg). The rising phase of aMT.6S excretion on the second subjective night is replotted on the third and fourth nights (blue lines) to emphasise the delay.
Characterisation of the receptor sub-type involved in this response is difficult due to the lack of high affinity, specific 5-HT2c antagonists. We approached this problem by taking advantage of 2 serotonin antagonists which have different affinity for the 5-HT2a and 5-HT2c receptors. Ritanserin has high affinity for both receptors whereas ketanserin is 10 fold less potent than ritanserin at 5-HT2c receptors. A range of equimolar doses of antagonists was administered prior to DOI and the inhibition of phase shifting determined (Kennaway and Moyer 1998) [10]. Figure 3 shows that ritanserin was more potent than ketanserin providing further circumstantial evidence for 5-HT2c involvement of DOI induced phase shifts.
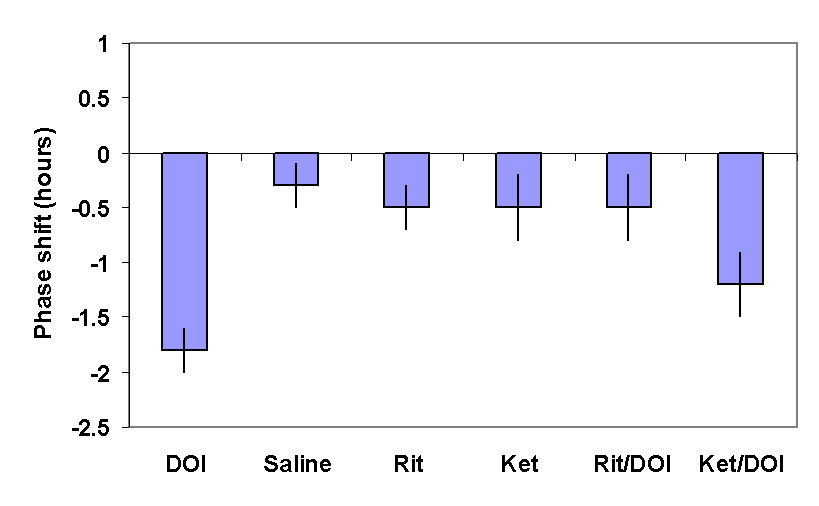
Figure 3. The mean
± SEM of the change in the time of onset of aMT.6S excretion provoked by DOI and its blockade by prior administration of ritanserin (0.07m mole/kg) but not ketanserin at the same dose.
Immunohistochemical studies of SCN 5-HT receptors
There have been many studies on the distribution of 5-HT receptors in the rat brain. Our search of the literature indicated that there was a large body of evidence indicating the presence of 5-HT2c receptors in the SCN [11-15] but surprisingly there has been little interest in the action of agonists for the receptor on rhythms. We have also recently conducted studies on the presence of 5-HT receptors using an immunocytochemical approach. As might be predicted from the in situ hybridisation studies [16,17], we were unable to detect 5-HT7 receptors in the rat SCN by immunohistochemistry (Moyer and Kennaway, manuscript in preparation). Scarce immuno-labelling was observed in the SCN when sections were incubated with anti-5HT2a antiserum (Figure 4). Examination at higher magnification showed few intensely stained fibres which travelled a very short distance within each section. These structures were uniformly distributed in the SCN and were also frequently observed in the optic chiasma (not shown). Sections incubated with antibodies against 5HT2c displayed a considerable amount of immunoreactivity located in the ventral portion of the SCN (fig. 4). At higher magnification, the SCN area showed diffuse immunoreactivity which delineated clear circular areas (not shown).
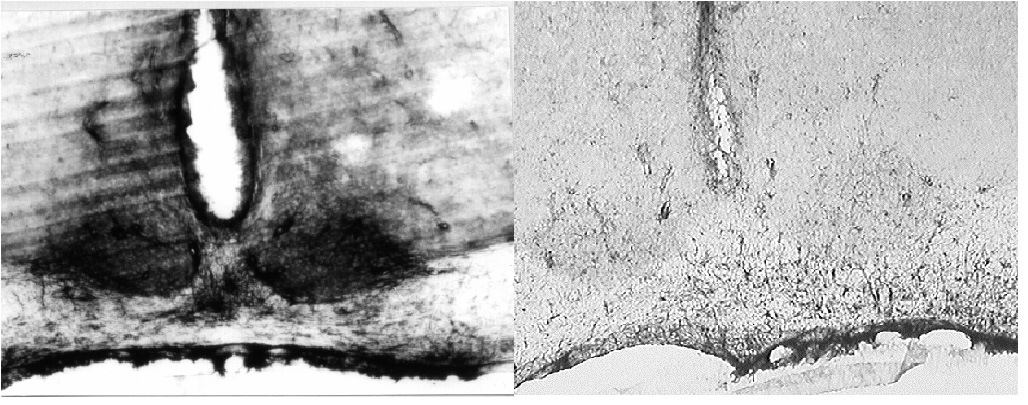
Figure 4
(a) A considerable amount of 5-HT2c - like immunoreactivity was observed in the ventral portion of the rat SCN. (b) 5-HT2a - like immunoreactivity in the SCN was considerably less conspicuous and is probably restricted to astrocytic processes.
Excitatory amino acid - serotonin interactions
The results obtained in our studies thus far might lead to the conclusion that a serotonergic pathway from the retina was the predominant route through which light altered rhythms. This conclusion could only be sustained if serotonin antagonists which block 5-HT2c receptors blocked light induced phase shifts. Neither metergoline (15 mg/kg), ritanserin (3 mg/kg) or LY 53,8757 (3 mg/kg) when administered 30 minutes before a 2 lux/1 min light pulse blocked the light induced phase delay or induction of c-fos [10]. Thus we concluded that there probably are at least 2 pathways operative and that one pathway remains patent during pharmacological blockade of the other. One of these other pathways is most likely the RHT utilising EEA neurotransmitters.
Our investigations of the interrelationship between EAA and serotonin pathways are in the early stages but we have recently obtained some extremely interesting results. As reported previously [6] prior administration of the NMDA antagonist MK-801 did not block the acute or phase shifting effects of a 2 lux/1 minute light pulse at CT18. When the same dose of MK-801 (3 mg/kg) was administered prior to DOI (0.5 mg/kg) at CT18, the delay in the onset of melatonin production on nights 3 and 4 was eliminated (figure 5a). Moreover when we investigated the effect of the drugs on the induction of c-fos in the rat SCN, MK-801 again blocked the stimulatory action of DOI (figure 5b). Thus it appears that the serotonin effects on rhythmicity involve a pathway that utilises EAA and NMDA receptors. Work is continuing to address the likely site of these neurons.
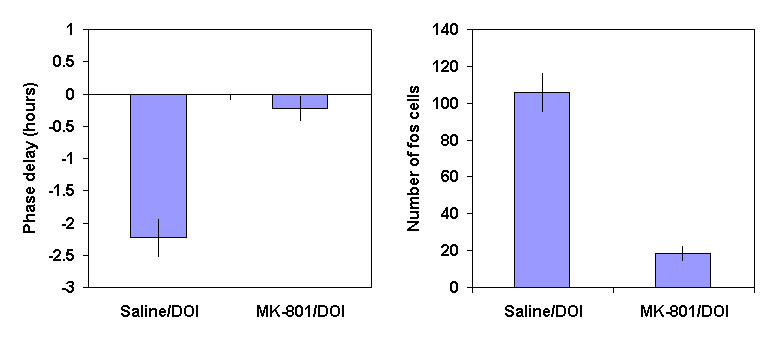
Figure 5 (a) The phase shift (mean
± SEM, hours) in the onset of aMT.6S excretion following administration of vehicle or MK-801 (3 mg/kg), 30 minutes before DOI (0.5 mg/kg) at CT18(b) The number of SCN cell nuclei expressing c-FOS immunoreactivity (mean
± SEM) after vehicle or MK-801 (3 mg/kg) 30 minutes before DOI (0.5 mg/kg) at CT18.
Acetylcholine
Thus far we have concentrated on serotonin and to a lesser extent EAA as mediators of light effects on rhythms, but there are a number of other transmitters that might also be candidates. In the case of acetylcholine, cholinergic projections from the brainstem and basal forebrain terminate in the SCN. The presence of muscarinic and nicotinic receptors and choline acetyltransferase in the SCN provide circumstantial evidence for a role in circadian rhythmicity. More direct evidence for a role was provided from experiments where the non-specific agonist carbachol was micro-injected into the rat SCN and caused phase shifts in the rhythm of pineal NAT activity similar to those provoked by light.
In a recent study in our laboratory, rats were injected with nicotine (1 mg/kg) or saline at various times of the night and the effect on aMT.6S excretion rate rhythmicity determined. When the nicotine was injected at CT14, there was no significant effect on the time of onset of melatonin production the next night (0.8
± 0.3 hour delay) and saline had no effect at CT16 (0.3 ± 0.1 hour delay). When nicotine was administered at CT16 or CT18 the onset of melatonin production on the following nights were delayed significantly by 1.7 ± 0.3 and 1.7 ± 0.2 hours respectively. A light pulse at CT16 (2 lux/1min) resulted in a delay of 2.3 ± 0.2 hours the following night. Nicotine administration also caused the induction of c-fos in the SCN in a dose and time dependent manner. These results implicate cholinergic pathways in the mediation of light induced shifts in rhythms in the rat.Conclusion
The control by light of melatonin and other biological rhythms is more complex than once thought. Our own results have implicated 3 different neurotransmitters in the control of one phenomenon in the rat. Where the transmitters and their neurons fit into the neuroanatomy of the circadian system is not yet known. The problem is even more complex when one considers that at least some of the results we obtained in our in vivo rat experiments are at variance with results from in vitro studies on neuronal firing rate rhythms by others. In some cases the differences are merely one of degree (the magnitude of the phase shifts are often 3 or more hours in the in vitro experiments compared to 1-3 hour shifts in vivo), but in other cases the direction of response is different (eg 8-OHDPAT and quipazine induced phase advances). When one then examines different species, the area again clouds since there is compelling evidence that serotonergic agonists for 5-HT1a receptors block light induced phase shifts and c-fos induction in hamsters [18,19]. There is clearly a long way to go before we understand how light alters rhythms.
Acknowledgments
Original studies in this laboratory and the preparation of this paper were supported by the National Health and Medical Research Council of Australia. We acknowledge the assistance of Shawn Rowe, Sarah Palnok, Athena Voultsios and Lisa Mazzone.
References
1. Shen, H. and Semba, K. , A direct retinal projection to the dorsal raphe nucleus in the rat, Brain Res. 635 (1994) 159-168.
2. Kennaway, D.J. , Urinary 6-Sulphatoxymelatonin excretory rhythms in laboratory rats - Effects of photoperiod and light, Brain Res. 603 (1993) 338-342.
3. Aschoff, J. , Response curves in circadian periodicity, J. Aschoff (Ed.) Circadian Clocks, North Holland Publishing, Amsterdam, 1965, pp. 95-111.
4. Kennaway, D. J. and Rowe, S. A. , Impact of light pulses on 6-sulphatoxymelatonin rhythms in rats, J. Pineal Res. 16 (1994) 65-72.
5. Illnerova, H. and Vanecek, J. , Entrainment of the circadian rhythm in the rat pineal N- acetyltransferase activity by prolonged periods of light. J. Comp Physiol. A 161 (1987) 495-510.
6. Rowe, S.A. and Kennaway, D.J. , Effect of NMDA receptor blockade on melatonin and activity rhythm responses to a light pulse in rats, Brain Res. Bull. 41 (1996) 351-358.
7. Kennaway, D. J. Light, neurotransmitters and the suprachiasmatic nucleus control of pineal melatonin production in the rat. Biological Signals 6 (1997) 247-254.
8. Kennaway, D.J., Rowe, S.A. and Ferguson, S.A. , Serotonin agonists mimic the phase shifting effects of light on the melatonin rhythm in rats, Brain Res. 737 (1996) 301-307.
9. Moyer, R.W., Kennaway, D.J., Ferguson, S.A. and Dijstelbloem, Y.P. , Quipazine and light have similar effects on c-fos induction in the rat suprachiasmatic nucleus, Brain Res, 765 (1997) 337-342.
10. Kennaway, D. J. and Moyer R. W. , Serotonin 5-HT2c agonists mimic the effect of light pulses on circadian rhythms. Brain Res. 806 (1998) 257-270.
11 Hoffman, B.J. and Mezey, E. , Distribution of serotonin 5-HT1C receptor mRNA in adult rat brain, FEBS Lett. 247 (1989) 453-462.
12 Holmes, M.C., French, K.L. and Seckl, J.R. , Dysregulation of diurnal rhythms of serotonin 5-HT2c and corticosteroid receptor gene expression in the hippocampus with food restriction and glucocorticods, J Neurosci. 17 (1997) 4056-4065.
13 Pompeiano, M., Palacios, J.M. and Mengod, G. , Distribution of the serotonin 5-HT2 receptor family mRNAs: comparison between 5-HT2A and 5-HT2C receptors, Brain Res. Mol. Brain Res. 23 (1994) 163-178.
14 Roca, A.L., Weaver, D.R. and Reppert, S.M. , Serotonin receptor gene expression in the rat suprachiasmatic nuclei, Brain Res. 608 (1993) 159-165.
15 Wright, D.E., Seroogy, K.B., Lundgren, K.H., Davis, B.M. and Jennes, L. , Comparative localization of serotonin1A, 1C, and 2 receptor subtype mRNAs in rat brain, J. Comp. Neurol. 351 (1995) 357-373.
16 Lovenberg, T.W., Baron, B.M., de-Lecea, L., Miller, J.D., Prosser, R.A., Rea, M.A., Foye, P.E., Racke, M., Slone, A.L., Siegel, B.W., Danielson, P.E., Sutcliffe, J.G. and Erlander, M.G. , A novel adenylyl cyclase-activating serotonin receptor (5-HT7) implicated in the regulation of mammalian circadian rhythms, Neuron, 11 (1993) 449-458.
17 Gustafson, E.L., Durkin, M.M., Bard, J.A., Zgombick, J. and Branchek, T.A. , A receptor autoradiographic and in situ hybridization analysis of the distribution of the 5-ht7 receptor in rat brain, Br. J. Pharmacol. 117 (1996) 657-666.
18. Glass, J.D., Selim, M. and Rea, M.A. , Modulation of light-induced C-Fos expression in the suprachiasmatic nuclei by 5-HT1A receptor agonists, Brain Res. 638 (1994) 235-242.
19. Rea, M.A., Glass, J.D. and Colwell, C.S. , Serotonin modulates photic responses in the hamster suprachiasmatic nuclei, J. Neurosci. 14 (1994) 3635-3642.
| Discussion Board | Previous Page | Your Symposium |