|
|
INABIS '98 Home Page |
|
Your Symposium |
|
Related Symposia & Posters |
|
Scientific Program |
|
Exhibitors' Foyer |
|
Personal Itinerary |
|
New Search |
The Impact of Male Sensory Cues on the Female Brain
K. M. Kendrick
INTRODUCTION
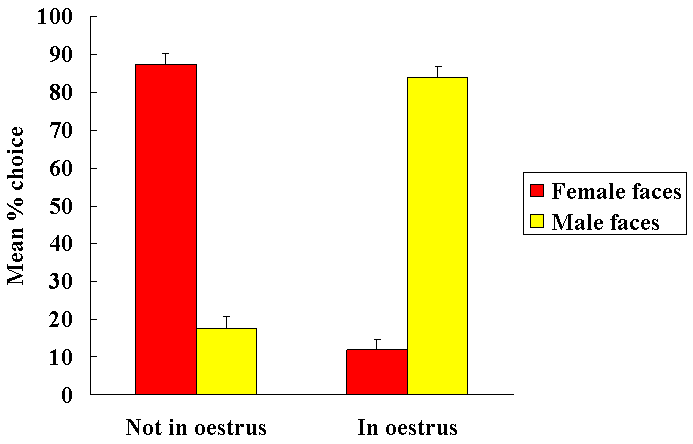
Female sheep, similar to other mammalian species, only show receptiveand proceptive sexual responses towards males when they are in oestrus.Sexual responsiveness during oestrus is stimulated by the feedback actionsof altered levels of progesterone and oestrogen on the brain particularlyat the level of the hypothalamic mediobasal hypothalamus. Evidence suggeststhat females find both visual (faces - see Fig. 1 Kendrick et al.,1995) and olfactory cues from males more attractive when they are in oestruscompared with when they are not. We have also shown that neurochemicalrelease in the mediobasal hypothalamus in response to pictures of malefaces or their odours only occurs when females are in oestrus (Fig. 2 -see Fabre-Nys et al., 1997). A central question therefore is theextent to which hormonal changes alter the impact of male sensory cueson the female brain that might explain her differential attraction andresponsiveness to males as a function of her reproductive cycle.
In a 2-choice maze sheep chose to approach female faces ratherthan male ones when not sexually receptive (Luteal), but do the oppositewhen they are receptive (Oestrus). (From Kendrick et al. 1995)
Male faces and odours only evoke noradrenaline release when femalesare in oestrus. Similarly male faces only evoke glutamate release at thistime. * P < 0.05 vs. baseline, + P <0.05 vs. male pen. (From Fabre-Nyset al. 1997).
We have found that quantification of activity-dependent changes in c-fosmRNA is a reliable method for determining differential patterns of brainactivation in the sheep in the same way as it has been shown in rodents.Quantification of message rather than protein changes has proved to bethe most reliable way of determining subtle changes in c-fos expressionin this species when one is considering physiological rather than pharmacologicallyevoked changes. This method has allowed us to quantify patterns of neuralactivation following birth and exposure to lambs (da Costa et al.,1997) and exposure to faces (Kendrick, 1998). This poster describes theapplication of this technique to address the question of how the female'sreproductive cycle alters the responsiveness of her brain to male visualand olfactory cues and to somatosensory stimulation during mating (seeOhkura et al., 1997).
METHODS
Animals and treatments
Sixteen, sexually experienced, ovariectomised adult female Clun Forestsheep were used. All the animals received a vaginal sponge containing 60mg of medroxyprogesterone acetate and a 1cm subcutaneous silastic tubingimplant containing oestradiol for 7 days to mimic circulating steroid levelsduring luteal phase of intact ewes. The ewes were randomly divided intofour groups of four animals and three groups received a 20 microgram oestradiolinjection (i.m.) 10 h after the removal of a vaginal sponge to induce oestrus.The remaining group received an oil vehicle control injection alone. Thefollowing day (16-20 h after oestradiol injection) each animal of one ofthe oestrus groups was exposed to a male for 5-min in a small 3m x 2m testingarea adjacent to their home pens and both mating and intromission tookplace; a second group had the same 5-min exposure to the male but intromissionwas prevented by the male wearing an apron. The third oestrus group wasexposed to the testing procedure but not to the male. Animals in the anoestrusgroup were also exposed to a male for 5 min. All animals were exposed tothe same duration of, and type of handling by, the experimenters. The amountof time the female looked in the male's direction during the tests wasmade and the number of mounts and intromissions received recorded. To avoidconfounding effects of events not related to the exposure to the male,tests were always conducted at the same time of day (14.00 to 16.00) andat least 7 hours after the animals had been fed.
All ewes were killed 30 min after exposure to a male (or at an equivalenttime in the group not exposed to a male) by an intravenous injection ofsodium pentobarbitone. The brains were rapidly removed in a sterile mannerand frozen ( -80oC) until sectioning.
In situ hybridization
Brains were cryostat-sectioned (12 microns thick) and every tenth section thaw-mounted onto glass slides coatedwith poly-L-lysine and air dried. Sections were fixed for 5 min at 4oCin 0.01 M phosphate-buffered saline (PBS, pH 7.4) containing 4% paraformaldehyde,washed three times in PBS for 2 min each, dehydrated in an ascending ethanolseries and stored in 95% ethanol at 4oC until use. A standard in situ hybridisation histochemistry protocol then was carried out for visualising c-fos mRNA using a cocktail of 3, 45mer c-fos antisenseoligonucleotide probes with sections laid down both on autoradiographicfilm, for macroautoradiography (3 weeks), and emulsion dipped for cellular grain counting (12 weeks).
Quantification of c-fos mRNA expression
Levels of c-fos gene expression were quantified using an imageanalysis system which could measure either measure optical densities on autoradiograpic film with reference to C14 standards or count the numberof silver grains over neuronal cells.
RESULTS
Female sexual behaviour during oestrus and anoestrus
Oestrus females remained immobile in response to male courtship behaviour and accepted mounting attempts. In the first oestrus group, several mounts(2-4) followed by a single intromission and ejaculation were observed during the 5-min test. Animals prevented from experiencing intromissions received the same number of mounts (3-6). Ewes in anoestrus were totally unreceptive to the male but nevertheless all received mounting attempts (1-3 per female).Since females were always <1.5m from the males they would have been exposed to similar amounts of olfactory stimulation from them irrespective of their oestrus state. Also, all females spent at least 75% of the 5 min test looking at the males
Effects of oestrus on c-fos mRNA expression following exposure to a male
C-fos mRNA expression levels were quantified in 29 brain regionsor nuclei throughout the brain by grain counting (TABLE 1). Some constitutive expression (defined as >30 grains per cell) was seen in all of the regions analysed and none of the treatments significantly altered the number of cells expressing c-fos mRNA.
| Brain Region | Oestrus+male+intromission (n=4) |
Oestrus+male (n=4) |
Oestrus (n=4) |
Anoestrus+male (n=4) |
|---|---|---|---|---|
| Primary sensory cortex | ||||
| Olfactory bulb | ||||
| Mitral cell layer | 156±16* | 172±13* | 109±5 | 124±7 |
| Granule cell layer | 91±9* | 108±5* | 62±3 | 65±7 |
| Visual (V1, V2) | 187±5* | 214±18* | 112±5 | 134±5 |
| Somatosensory | 226±13** | 241±27** | 106±2 | 119±15 |
| Association cortex | ||||
| Anterior cortex | 349±30** | 269±29** | 99±12 | 127±17 |
| Temporal | 297±28** | 291±20** | 123±7 | 166±12† |
| Orbitofrontal | 170±10* | 194±14** | 119±6 | 128±5 |
| Piriform | 226±29* | 179±13 | 144±10 | 108±13 |
| Entorhinal | 77±10 | 86±11 | 63±8 | 68±9 |
| Thalamus | ||||
| Lateral gen. Nucleus | 123±8 | 119±11 | 99±5 | 69±9 |
| Mediodorsal nucleus | 133±14* | 129±10* | 66±8 | 75±3 |
| Limbic system | ||||
| BNST | 153±4** | 139±15** | 64±4 | 75±8 |
| Lateral septum | 130±21 | 169±14* | 88±5 | 95±10 |
| Basolateral amygdala | 235±41 | 223±40* | 88±18 | 95±19 |
| Medial amygdala | 197±41 | 212±14* | 117±17 | 98±12v |
| Lateral amygdala | 279±36** | 279±33** | 105±22 | 94±21 |
| Hippocampus | ||||
| CA1 | 160±10** | 160±9** | 88±4 | 119±13 |
| CA2 | 129±11 | 121±10 | 127±6 | 108±2 |
| CA3 | 139±15 | 115±3 | 100±5 | 128±15 |
| CA4 | 144±12 | 143±12 | 114±8 | 127±6 |
| Dentate gyrus | 36±4 | 41±3+ | 32±1 | 26±2 |
| Subiculum | 199±20** | 167±6* | 101±12 | 121±5 |
| Basal ganglia | ||||
| Nacc | 145±14* | 138±10* | 85±10 | 91±10 |
| Striatum | 54±6 | 50±8 | 45±5 | 40±5 |
| Brainstem | ||||
| Nuc. of solitary tract | 83±6+ | 77±5+ | 61±7 | 44±7 |
| Hypothalamus | ||||
| mPOA | 275±21** | 206±17** | 88±17 | 78±21 |
| MBH | 262±29** | 222±17** | 101±6 | 78±15 |
| PVN | 168±23** | 154±3** | 76±3 | 68±5 |
| Supraoptic nucleus | 120±3+ | 102±8 | 99±6 | 84±10v |
P<0.05, ** P<0.01 two-tailed (Tukey-Kramer test )vs. oestrus and anoestrus+male groups, + P<0.05 vs. anoestrus group, † P<0.05 vs.oestrus. (Numbers are mean number of silver grains per cell)
Overall levels of c-fos mRNA expression in the oestrus groupexposed to testing environment, but not to the male, and in the anoestrusgroup exposed to the male were comparable and extremely low and only inthe temporal cortex, which is important for face recognition, was therea small significant increase in the anoestrus group. The oestrus animalsexposed to a male showed significantly higher levels of c-fos mRNAexpression in the primary sensory cortical regions for olfaction (mitraland granule cell layer of the olfactory bulb), vision (visual cortex) andtouch (somatosensory cortex ). Increased expression was also seen in associationcortical areas (cingulate, orbitofrontal, piriform and temporal cortices),limbic system (bed nucleus of the stria terminalis -BNST, lateral septum,basolateral amygdala, lateral amygdala, subiculum and hippocampus CA1),nucleus accumbens, hypothalamus (medial preoptic area, mediobasal hypothalamus- MBH) and brainstem (nucleus of the solitary tract).
Dark field autoradiographs showing c-fos mRNA expression in theorbitofrontal cortex (ORB CX), olfactory bulb (OB), temporal cortex (TCX- important for face recognition in sheep), medial preoptic area (MPOA),mediobasal hypothalamus (MBH), and medial (MA), lateral (LA), and basolateral(BLA) amygdala.
Effects of intromission on c-fos expression
In contrast to rodent experiments, we could find no obvious enhancementof c-fos mRNA expression in females that had received an intromissionand ejaculation.
CONCLUSIONS
- When sexually receptive females are exposed to male sensory cues thereis extensive activation (as evidenced by altered c-fos mRNA expression)of primary sensory and association cortical regions associated with visualand olfactory processing. There is also strong activation in limbic, hypothalamicand basal ganglia regions associated with the female sexual response andreward.
- When females are not sexually receptive, exposure to the same sensorycues from the male has virtually no impact on brain activation at all irrespectiveof whether the brain regions contain sex hormone-concentrating cells ornot.
- This evidence from c-fos mapping is supported by in vivoneurochemical experiments showing that pictures of male faces only evoketransmitter release from the mediobasal hypothalamus when females are sexuallyreceptive.
- The patterns of c-fos expression we have seen occur independentof whether the male achieves intromissions and ejaculates. Differencesbetween sheep and rats in this respect may reflect the fact that sheeponly experience a single intromission prior to ejaculation whereas ratsexperience multiple ones.
- Hormonal changes per se during the oestrus cycle do not appearto influence basal levels of c-fos mRNA expression.
- These results provide further support for the use of c-fos mRNAas a functional marker for neural activation. However, results must alwaysbe qualified by the fact that not all cells in the brain express c-fos,and therefore other brain regions may have been activated that we couldnot detect. The technique also cannot detect cells whose activity has beeninhibited by the stimuli. This might be of particular relevance when consideringour anoestrus animals since in these individuals the impact of male sensorycues might have been predominantly inhibitory rather than simply a reflectionof a lack of activation.
REFERENCES
Da Costa, A.P.C., Broad, K.D. and Kendrick, K.M. (1997) Olfactory memoryand maternal behaviour-induced changes in c-fos and zif/268mRNA expression in sheep brain. Molecular Brain Research 46:63-76
Fabre-Nys, C., Ohkura, S. and Kendrick, K.M. (1997) Male faces and odoursevoke differential patterns of neurochemical release in the mediobasalhypothalamus of the ewe during oestrus: an insight into sexual motivation?European Journal of Neuroscience 9:1666-1677.
Kendrick, K.M. (1998) Intelligent perception. Applied Behaviour AnimalScience 57:213-231.
Kendrick, K.M., Atkins, K., Hinton, M.R., Broad, K.D., Fabre-Nys, C.and Keverne E.B. (1995). Facial and vocal discrimination in sheep. AnimalBehaviour 49:1665-1676.
Ohkura, S., Fabre-Nys, C., Broad, K.D. and Kendrick, K.M. (1997) Sexhormones enhance the impact of male sensory cues on both primary and associationcortical components of visual and olfactory processing pathways as wellas in limbic and hypothalamic regions in female sheep. Neuroscience80:285-297.
 click to enlarge
click to enlarge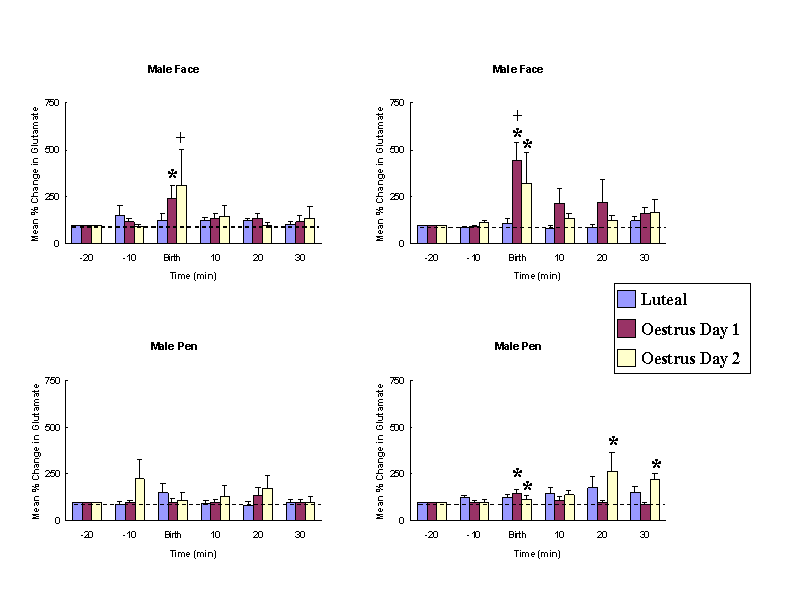 click to enlarge
click to enlarge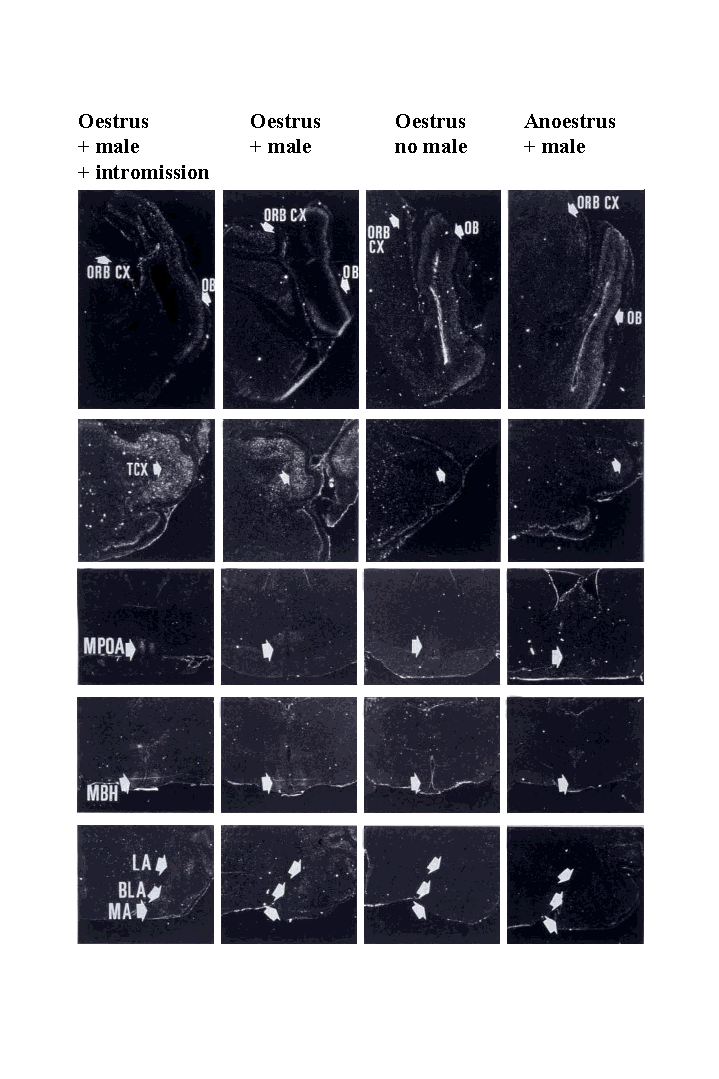 click to enlarge
click to enlarge